GRIA2/ENPP3 Regulates the Proliferation and Migration of Vascular Smooth Muscle Cells in the Restenosis Process Post-PTA in Lower Extremity Arteries
- PMID: 34504438
- PMCID: PMC8423086
- DOI: 10.3389/fphys.2021.712400
GRIA2/ENPP3 Regulates the Proliferation and Migration of Vascular Smooth Muscle Cells in the Restenosis Process Post-PTA in Lower Extremity Arteries
Abstract
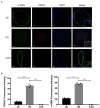
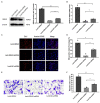
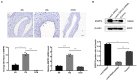
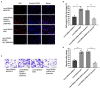
Restenosis is the main restriction on the long-term efficacy of percutaneous transluminal angioplasty (PTA) therapy for peripheral artery disease (PAD). Interventions to prevent restenosis are poor, and the exact mechanism is unclear. Here, we aimed to elucidate the role of GRIA2 in the restenosis process post-PTA in lower extremity arteries. We searched the differentially expressed genes (DEGs) between atherosclerotic and restenotic artery plaques in the Gene Expression Omnibus (GEO), and five DEGs were identified. Combined with Gene Ontology (GO) enrichment analysis, GRIA2 was significantly correlated with the restenosis process. Tissue samples were used to examine GRIA2 expression by immunofluorescence staining of atherosclerotic and restenotic artery plaques. The regulation of GRIA2 in vascular smooth muscle cells (VSMCs) was confirmed by lentiviral transfection. Overexpression of GRIA2 promoted the proliferation and migration of VSMCs. Using Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analysis and protein-protein interaction (PPI) network, a strong connection between ENPP3 and GRIA2 was discovered. In vitro results showed that the high expression of GRIA2 in VSMCs enhanced the expression of ENPP3, while downregulation of GRIA2 downregulated ENPP3. GRIA2 is highly differentially expressed in restenotic arterial plaques, promoting the proliferation and migration of VSMCs through upregulation of ENPP3. These discoveries will help us to obtain a better understanding of restenosis in lower extremity arteries.
Keywords: GRIA2; percutaneous transluminal angioplasty; peripheral artery disease; restenosis; vascular smooth muscle cells.
Copyright © 2021 Zhou, Qi and Gu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be constructed as a potential conflict of interest.
Figures

Similar articles
-
Lin28a up-regulation is associated with the formation of restenosis via promoting proliferation and migration of vascular smooth muscle cells.J Cell Mol Med. 2020 Sep;24(17):9682-9691. doi: 10.1111/jcmm.15506. Epub 2020 Jul 25. J Cell Mol Med. 2020. PMID: 32710472 Free PMC article.
-
Identification of a Novel Gene Correlated With Vascular Smooth Muscle Cells Proliferation and Migration in Chronic Thromboembolic Pulmonary Hypertension.Front Physiol. 2021 Nov 11;12:744219. doi: 10.3389/fphys.2021.744219. eCollection 2021. Front Physiol. 2021. PMID: 34858201 Free PMC article.
-
Randomized controlled trial of aspirin and clopidogrel versus aspirin and placebo on markers of smooth muscle proliferation before and after peripheral angioplasty.J Vasc Surg. 2009 Oct;50(4):861-9. doi: 10.1016/j.jvs.2009.06.017. J Vasc Surg. 2009. PMID: 19786240 Clinical Trial.
-
Intravascular brachytherapy for peripheral vascular disease.Cochrane Database Syst Rev. 2002;(4):CD003504. doi: 10.1002/14651858.CD003504. Cochrane Database Syst Rev. 2002. Update in: Cochrane Database Syst Rev. 2014 Jan 08;(1):CD003504. doi: 10.1002/14651858.CD003504.pub2 PMID: 12519600 Updated. Review.
-
Preventing restenosis after angioplasty: a multistage approach.Clin Sci (Lond). 2008 Feb;114(4):257-64. doi: 10.1042/CS20070228. Clin Sci (Lond). 2008. PMID: 18194134 Review.
Cited by
-
Hypomethylation of the ENPP3 promoter region contributes to the occurrence and development of ovarian endometriosis via the AKT/mTOR/4EBP1 signaling pathway.Biomol Biomed. 2023 Dec 27;24(4):848-856. doi: 10.17305/bb.2023.9989. Biomol Biomed. 2023. PMID: 38149831 Free PMC article.
-
Metabolomics revealed pharmacodynamic effects of aspirin and indobufen in patients after percutaneous transluminal angioplasty surgery.Front Cardiovasc Med. 2024 Oct 29;11:1433643. doi: 10.3389/fcvm.2024.1433643. eCollection 2024. Front Cardiovasc Med. 2024. PMID: 39534497 Free PMC article.
-
Identification of immune-related molecular markers in intracranial aneurysm (IA) based on machine learning and cytoscape-cytohubba plug-in.BMC Genom Data. 2023 Apr 11;24(1):20. doi: 10.1186/s12863-023-01121-w. BMC Genom Data. 2023. PMID: 37041519 Free PMC article.
References
-
- Boggavarapu N. R., Lalitkumar S., Joshua V., Kasvandik S., Salumets A., Lalitkumar P. G., et al. . (2016). Compartmentalized gene expression profiling of receptive endometrium reveals progesterone regulated ENPP3 is differentially expressed and secreted in glycosylated form. Sci. Rep. 6:33811. 10.1038/srep33811, PMID: - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

