Beyond the Driver Mutation: Immunotherapies in Gastrointestinal Stromal Tumors
- PMID: 34489967
- PMCID: PMC8417712
- DOI: 10.3389/fimmu.2021.715727
Beyond the Driver Mutation: Immunotherapies in Gastrointestinal Stromal Tumors
Abstract
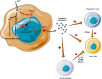
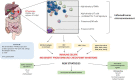
Gastrointestinal stromal tumors (GISTs) are a subtype of soft tissue sarcoma (STS), and have become a concept of oncogenic addiction and targeted therapies.The large majority of these tumors develop after a mutation in KIT or platelet derived growth factor receptor α (PDGFRα), resulting in uncontrolled proliferation. GISTs are highly sensitive to imatinib. GISTs are immune infiltrated tumors with a predominance of tumor-associated macrophages (TAMs) and T-cells, including many CD8+ T-cells, whose numbers are prognostic. The genomic expression profile is that of an inhibited Th1 response and the presence of tertiary lymphoid structures and B cell signatures, which are known as predictive to response to ICI. However, the microtumoral environment has immunosuppressive attributes, with immunosuppressive M2 macrophages, overexpression of indoleamine 2,3-dioxygenase (IDO) or PD-L1, and loss of major histocompatibility complex type 1. In addition to inhibiting the KIT oncogene, imatinib appears to act by promoting cytotoxic T-cell activity, interacting with natural killer cells, and inhibiting the expression of PD-L1. Paradoxically, imatinib also appears to induce M2 polarization of macrophages. There have been few immunotherapy trials with anti-CTLA-4 or anti-PD-L1drugs and available clinical data are not very promising. Based on this comprehensive analysis of TME, we believe three immunotherapeutic strategies must be underlined in GIST. First, patients included in clinical trials must be better selected, based on the identified driver mutation (such as PDGFRα D842V mutation), the presence of tertiary lymphoid structures (TLS) or PD-L1 expression. Moreover, innovative immunotherapeutic agents also provide great interest in GIST, and there is a strong rationale for exploring IDO targeting after disease progression during imatinib therapy. Finally and most importantly, there is a strong rationale to combine of c-kit inhibition with immune checkpoint inhibitors.
Keywords: GIST - gastro intestinal stromal tumor; IDO - indoleamine 2,3-dioxygenase; KIT; PD-L1; imatinib; immunologic response; immunotherapy; macrophages (M1/M2).
Copyright © 2021 Roulleaux Dugage, Jones, Trent, Champiat and Dumont.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Improving Immunotherapy Efficacy in Soft-Tissue Sarcomas: A Biomarker Driven and Histotype Tailored Review.Front Immunol. 2021 Dec 3;12:775761. doi: 10.3389/fimmu.2021.775761. eCollection 2021. Front Immunol. 2021. PMID: 34925348 Free PMC article. Review.
-
PD-1/PD-L1 Blockade Enhances T-cell Activity and Antitumor Efficacy of Imatinib in Gastrointestinal Stromal Tumors.Clin Cancer Res. 2017 Jan 15;23(2):454-465. doi: 10.1158/1078-0432.CCR-16-1163. Epub 2016 Jul 28. Clin Cancer Res. 2017. PMID: 27470968 Free PMC article.
-
Current status of immunotherapy for gastrointestinal stromal tumor.Cancer Gene Ther. 2017 Mar;24(3):130-133. doi: 10.1038/cgt.2016.58. Epub 2017 Feb 10. Cancer Gene Ther. 2017. PMID: 28186088 Review.
-
Latest advances in adult gastrointestinal stromal tumors.Future Oncol. 2017 Oct;13(24):2183-2193. doi: 10.2217/fon-2017-0245. Epub 2017 Oct 6. Future Oncol. 2017. PMID: 28984483 Review.
-
Role of immune microenvironment in gastrointestinal stromal tumours.Histopathology. 2018 Feb;72(3):405-413. doi: 10.1111/his.13382. Epub 2017 Nov 21. Histopathology. 2018. PMID: 28871595
Cited by
-
The Prognostic Value of Plasma Programmed Death Protein-1 (PD-1) and Programmed Death-Ligand 1 (PD-L1) in Patients with Gastrointestinal Stromal Tumor.Cancers (Basel). 2022 Nov 23;14(23):5753. doi: 10.3390/cancers14235753. Cancers (Basel). 2022. PMID: 36497235 Free PMC article.
-
Unexpected reaction of "wild-type" gastrointestinal stromal tumor to imatinib: case report and literature review.Front Oncol. 2024 Jan 31;13:1334784. doi: 10.3389/fonc.2023.1334784. eCollection 2023. Front Oncol. 2024. PMID: 38357425 Free PMC article.
-
Novel Hypoxia-Associated Gene Signature Depicts Tumor Immune Microenvironment and Predicts Prognosis of Colon Cancer Patients.Front Genet. 2022 Jun 6;13:901734. doi: 10.3389/fgene.2022.901734. eCollection 2022. Front Genet. 2022. PMID: 35734431 Free PMC article.
-
Advances in immunology and immunotherapy for mesenchymal gastrointestinal cancers.Mol Cancer. 2023 Apr 18;22(1):71. doi: 10.1186/s12943-023-01770-6. Mol Cancer. 2023. PMID: 37072770 Free PMC article. Review.
-
KIT mutations and expression: current knowledge and new insights for overcoming IM resistance in GIST.Cell Commun Signal. 2024 Feb 27;22(1):153. doi: 10.1186/s12964-023-01411-x. Cell Commun Signal. 2024. PMID: 38414063 Free PMC article. Review.
References
-
- Ducimetière F, Lurkin A, Ranchère-Vince D, Decouvelaere A-V, Péoc’h M, Istier L, et al. . Incidence of Sarcoma Histotypes and Molecular Subtypes in a Prospective Epidemiological Study With Central Pathology Review and Molecular Testing. PloS One (2011) 6(8):e20294. 10.1371/journal.pone.0020294 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

