Who let the DoGs out? - biogenesis of stress-induced readthrough transcripts
- PMID: 34489151
- PMCID: PMC8840951
- DOI: 10.1016/j.tibs.2021.08.003
Who let the DoGs out? - biogenesis of stress-induced readthrough transcripts
Abstract
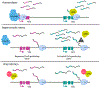
Readthrough transcription caused by inefficient 3'-end cleavage of nascent mRNAs has emerged as a hallmark of the mammalian cellular stress response and results in the production of long noncoding RNAs known as downstream-of-gene (DoG)-containing transcripts. DoGs arise from around 10% of human protein-coding genes and are retained in the nucleus. They are produced minutes after cell exposure to stress and can be detected hours after stress removal. However, their biogenesis and the role(s) that DoGs or their production play in the cellular stress response are incompletely understood. We discuss findings that implicate host and viral proteins in the mechanisms underlying DoG production, as well as the transcriptional landscapes that accompany DoG induction under different stress conditions.
Keywords: 3′-end cleavage; cellular stress; long noncoding RNAs; readthrough transcription; transcription termination.
Copyright © 2021 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no conflicts of interest.
Figures

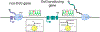
Similar articles
-
Remarkable improvement in detection of readthrough downstream-of-gene transcripts by semi-extractable RNA-sequencing.RNA. 2023 Feb;29(2):170-177. doi: 10.1261/rna.079469.122. Epub 2022 Nov 16. RNA. 2023. PMID: 36384963 Free PMC article.
-
It's a DoG-eat-DoG world-altered transcriptional mechanisms drive downstream-of-gene (DoG) transcript production.Mol Cell. 2022 Jun 2;82(11):1981-1991. doi: 10.1016/j.molcel.2022.04.008. Epub 2022 Apr 28. Mol Cell. 2022. PMID: 35487209 Free PMC article. Review.
-
Readthrough transcription: How are DoGs made and what do they do?RNA Biol. 2017 May 4;14(5):632-636. doi: 10.1080/15476286.2016.1149680. Epub 2016 Feb 9. RNA Biol. 2017. PMID: 26861889 Free PMC article. Review.
-
Transcription start sites at the end of protein-coding genes.Hum Genomics. 2018 Mar 16;12(1):15. doi: 10.1186/s40246-018-0146-6. Hum Genomics. 2018. PMID: 29548296 Free PMC article.
-
Comparative analysis reveals genomic features of stress-induced transcriptional readthrough.Proc Natl Acad Sci U S A. 2017 Oct 3;114(40):E8362-E8371. doi: 10.1073/pnas.1711120114. Epub 2017 Sep 19. Proc Natl Acad Sci U S A. 2017. PMID: 28928151 Free PMC article.
Cited by
-
Remarkable improvement in detection of readthrough downstream-of-gene transcripts by semi-extractable RNA-sequencing.RNA. 2023 Feb;29(2):170-177. doi: 10.1261/rna.079469.122. Epub 2022 Nov 16. RNA. 2023. PMID: 36384963 Free PMC article.
-
Transcriptional Stress Induces the Generation of DoGs in Cancer Cells.Noncoding RNA. 2024 Jan 10;10(1):5. doi: 10.3390/ncrna10010005. Noncoding RNA. 2024. PMID: 38250805 Free PMC article.
-
Functional Assessment of a New PBX1 Variant in a 46,XY Fetus with Severe Syndromic Difference of Sexual Development through CRISPR-Cas9 Gene Editing.Genes (Basel). 2023 Jan 20;14(2):273. doi: 10.3390/genes14020273. Genes (Basel). 2023. PMID: 36833200 Free PMC article.
-
Stress-induced transcriptional readthrough into neighboring genes is linked to intron retention.iScience. 2022 Nov 9;25(12):105543. doi: 10.1016/j.isci.2022.105543. eCollection 2022 Dec 22. iScience. 2022. PMID: 36505935 Free PMC article.
-
It's a DoG-eat-DoG world-altered transcriptional mechanisms drive downstream-of-gene (DoG) transcript production.Mol Cell. 2022 Jun 2;82(11):1981-1991. doi: 10.1016/j.molcel.2022.04.008. Epub 2022 Apr 28. Mol Cell. 2022. PMID: 35487209 Free PMC article. Review.
References
-
- Richter K et al. (2010) The heat shock response: life on the verge of death. Mol Cell 40 (2), 253–66. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

