ImmTOR nanoparticles enhance AAV transgene expression after initial and repeat dosing in a mouse model of methylmalonic acidemia
- PMID: 34485611
- PMCID: PMC8399083
- DOI: 10.1016/j.omtm.2021.06.015
ImmTOR nanoparticles enhance AAV transgene expression after initial and repeat dosing in a mouse model of methylmalonic acidemia
Abstract
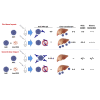
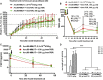
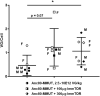
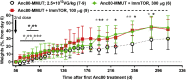
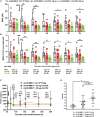
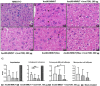
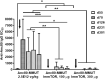
A major barrier to adeno-associated virus (AAV) gene therapy is the inability to re-dose patients due to formation of vector-induced neutralizing antibodies (Nabs). Tolerogenic nanoparticles encapsulating rapamycin (ImmTOR) provide long-term and specific suppression of adaptive immune responses, allowing for vector re-dosing. Moreover, co-administration of hepatotropic AAV vectors and ImmTOR leads to an increase of transgene expression even after the first dose. ImmTOR and AAV Anc80 encoding the methylmalonyl-coenzyme A (CoA) mutase (MMUT) combination was tested in a mouse model of methylmalonic acidemia, a disease caused by mutations in the MMUT gene. Repeated co-administration of Anc80 and ImmTOR was well tolerated and led to nearly complete inhibition of immunoglobulin (Ig)G antibodies to the Anc80 capsid. A more profound decrease of plasma levels of the key toxic metabolite, plasma methylmalonic acid (pMMA), and disease biomarker, fibroblast growth factor 21 (FGF21), was observed after treatment with the ImmTOR and Anc80-MMUT combination. In addition, there were higher numbers of viral genomes per cell (vg/cell) and increased transgene expression when ImmTOR was co-administered with Anc80-MMUT. These effects were dose-dependent, with the higher doses of ImmTOR providing higher vg/cell and mRNA levels, and an improved biomarker response. Combining of ImmTOR and AAV can not only block the IgG response against capsid, but it also appears to potentiate transduction and enhance therapeutic transgene expression in the mouse model.
Keywords: ImmTOR rapamycin-encapsulated nanoparticles; gene therapy; immunogenicity mitigation; re-dosing.
Conflict of interest statement
P.O.I., A.M.M., G.L.R., C.J.R., S.S.L., S.L.E., T.C., A.C., L.P.M.J., and T.K.K. are employees and shareholders of Selecta Biosciences. E.A.-M is an employee and holds stocks of Akouos, Inc. L.H.V. received consulting fees and research funding from Selecta Biosciences and holds equity in and serves on the Scientific Advisory Board of Akouos. He is an inventor of Anc80L65, licensed to biopharmaceutical companies, including Selecta Biosciences, from which he receives royalties. C.P.V. received research funding from Selecta Biosciences. R.J.C., L.L., I.M., and C.P.V. are co-inventors on patents and patent applications filed by the NIH on their behalf.
Figures

Similar articles
-
Enhancement of liver-directed transgene expression at initial and repeat doses of AAV vectors admixed with ImmTOR nanoparticles.Sci Adv. 2021 Feb 24;7(9):eabd0321. doi: 10.1126/sciadv.abd0321. Print 2021 Feb. Sci Adv. 2021. PMID: 33627416 Free PMC article.
-
Readministration of high-dose adeno-associated virus gene therapy vectors enabled by ImmTOR nanoparticles combined with B cell-targeted agents.PNAS Nexus. 2023 Nov 14;2(11):pgad394. doi: 10.1093/pnasnexus/pgad394. eCollection 2023 Nov. PNAS Nexus. 2023. PMID: 38024395 Free PMC article.
-
Promoterless, Nuclease-Free Genome Editing Confers a Growth Advantage for Corrected Hepatocytes in Mice With Methylmalonic Acidemia.Hepatology. 2021 Jun;73(6):2223-2237. doi: 10.1002/hep.31570. Epub 2021 May 21. Hepatology. 2021. PMID: 32976669 Free PMC article.
-
Development of ImmTOR Tolerogenic Nanoparticles for the Mitigation of Anti-drug Antibodies.Front Immunol. 2020 May 20;11:969. doi: 10.3389/fimmu.2020.00969. eCollection 2020. Front Immunol. 2020. PMID: 32508839 Free PMC article. Review.
-
Treatment of metabolic disorders using genomic technologies: Lessons from methylmalonic acidemia.J Inherit Metab Dis. 2022 Sep;45(5):872-888. doi: 10.1002/jimd.12534. Epub 2022 Jul 21. J Inherit Metab Dis. 2022. PMID: 35766386 Review.
Cited by
-
Engineering metabolism to modulate immunity.Adv Drug Deliv Rev. 2024 Jan;204:115122. doi: 10.1016/j.addr.2023.115122. Epub 2023 Nov 5. Adv Drug Deliv Rev. 2024. PMID: 37935318 Free PMC article. Review.
-
Liver directed adeno-associated viral vectors to treat metabolic disease.J Inherit Metab Dis. 2024 Jan;47(1):22-40. doi: 10.1002/jimd.12637. Epub 2023 Jun 5. J Inherit Metab Dis. 2024. PMID: 37254440 Free PMC article. Review.
-
Gene therapy for liver diseases - progress and challenges.Nat Rev Gastroenterol Hepatol. 2023 May;20(5):288-305. doi: 10.1038/s41575-022-00729-0. Epub 2023 Jan 16. Nat Rev Gastroenterol Hepatol. 2023. PMID: 36646909 Review.
-
Rescue of infant progressive familial intrahepatic cholestasis type 3 mice by repeated dosing of AAV gene therapy.JHEP Rep. 2023 Feb 24;5(5):100713. doi: 10.1016/j.jhepr.2023.100713. eCollection 2023 May. JHEP Rep. 2023. PMID: 37096142 Free PMC article.
-
B cell focused transient immune suppression protocol for efficient AAV readministration to the liver.Mol Ther Methods Clin Dev. 2024 Feb 20;32(1):101216. doi: 10.1016/j.omtm.2024.101216. eCollection 2024 Mar 14. Mol Ther Methods Clin Dev. 2024. PMID: 38440160 Free PMC article.
References
-
- de Baulny H.O., Benoist J.F., Rigal O., Touati G., Rabier D., Saudubray J.M. Methylmalonic and propionic acidaemias: Management and outcome. J. Inherit. Metab. Dis. 2005;28:415–423. - PubMed
-
- Manoli I., Sloan J.L., Venditti C.P. In: GeneReviews. Adam M.P., Ardinger H.H., Pagon R.A., Wallace S.E., Bean L.J.H., Stephens K., Amemiya A., editors. University of Washington, Seattle; 2005. Isolated methylmalonic acidemia; pp. 1993–2020. [Internet] - PubMed
-
- Hörster F., Baumgartner M.R., Viardot C., Suormala T., Burgard P., Fowler B., Hoffmann G.F., Garbade S.F., Kölker S., Baumgartner E.R. Long-term outcome in methylmalonic acidurias is influenced by the underlying defect (mut0, mut−, cblA, cblB) Pediatr. Res. 2007;62:225–230. - PubMed
-
- Kölker S., Valayannopoulos V., Burlina A.B., Sykut-Cegielska J., Wijburg F.A., Teles E.L., Zeman J., Dionisi-Vici C., Barić I., Karall D. The phenotypic spectrum of organic acidurias and urea cycle disorders. Part 2: The evolving clinical phenotype. J. Inherit. Metab. Dis. 2015;38:1059–1074. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

