In vivo Roles of Rab27 and Its Effectors in Exocytosis
- PMID: 34483204
- PMCID: PMC10511049
- DOI: 10.1247/csf.21043
In vivo Roles of Rab27 and Its Effectors in Exocytosis
Abstract
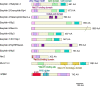
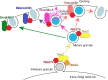
The monomeric GTPase Rab27 regulates exocytosis of a broad range of vesicles in multicellular organisms. Several effectors bind GTP-bound Rab27a and/or Rab27b on secretory vesicles to execute a series of exocytic steps, such as vesicle maturation, movement along microtubules, anchoring within the peripheral F-actin network, and tethering to the plasma membrane, via interactions with specific proteins and membrane lipids in a local milieu. Although Rab27 effectors generally promote exocytosis, they can also temporarily restrict it when they are involved in the rate-limiting step. Genetic alterations in Rab27-related molecules cause discrete diseases manifesting pigment dilution and immunodeficiency, and can also affect common diseases such as diabetes and cancer in complex ways. Although the function and mechanism of action of these effectors have been explored, it is unclear how multiple effectors act in coordination within a cell to regulate the secretory process as a whole. It seems that Rab27 and various effectors constitutively reside on individual vesicles to perform consecutive exocytic steps. The present review describes the unique properties and in vivo roles of the Rab27 system, and the functional relationship among different effectors coexpressed in single cells, with pancreatic beta cells used as an example.Key words: membrane trafficking, regulated exocytosis, insulin granules, pancreatic beta cells.
Keywords: insulin granules; membrane trafficking; pancreatic beta cells; regulated exocytosis.
Figures

Similar articles
-
The roles of Rab27 and its effectors in the regulated secretory pathways.Cell Struct Funct. 2003 Oct;28(5):465-74. doi: 10.1247/csf.28.465. Cell Struct Funct. 2003. PMID: 14745138 Review.
-
Rab27a and Rab27b regulate neutrophil azurophilic granule exocytosis and NADPH oxidase activity by independent mechanisms.Traffic. 2010 Apr;11(4):533-47. doi: 10.1111/j.1600-0854.2009.01029.x. Epub 2009 Dec 17. Traffic. 2010. PMID: 20028487 Free PMC article.
-
Rab27b is expressed in a wide range of exocytic cells and involved in the delivery of secretory granules near the plasma membrane.Mol Biol Cell. 2007 Nov;18(11):4377-86. doi: 10.1091/mbc.e07-05-0409. Epub 2007 Aug 29. Mol Biol Cell. 2007. PMID: 17761531 Free PMC article.
-
Multiple pathways and independent functional pools in insulin granule exocytosis.Genes Cells. 2023 Jul;28(7):471-481. doi: 10.1111/gtc.13029. Epub 2023 Apr 18. Genes Cells. 2023. PMID: 37070774 Free PMC article. Review.
-
The Rab-binding protein Noc2 is associated with insulin-containing secretory granules and is essential for pancreatic beta-cell exocytosis.Mol Endocrinol. 2004 Jan;18(1):117-26. doi: 10.1210/me.2003-0300. Epub 2003 Oct 30. Mol Endocrinol. 2004. PMID: 14593078
Cited by
-
Munc13b stimulus-dependently accumulates on granuphilin-mediated, docked granules prior to fusion.Cell Struct Funct. 2022 May 21;47(1):31-41. doi: 10.1247/csf.22005. Epub 2022 Apr 6. Cell Struct Funct. 2022. PMID: 35387942 Free PMC article.
-
Osh-dependent and -independent Regulation of PI4P Levels During Polarized Growth of Saccharomyces cerevisiae.Mol Biol Cell. 2023 Oct 1;34(11):ar104. doi: 10.1091/mbc.E23-03-0089. Epub 2023 Aug 9. Mol Biol Cell. 2023. PMID: 37556206 Free PMC article.
-
[Silencing RAB27a inhibits proliferation, invasion and adhesion of triple-negative breast cancer cells].Nan Fang Yi Ke Da Xue Xue Bao. 2023 Apr 20;43(4):560-567. doi: 10.12122/j.issn.1673-4254.2023.04.08. Nan Fang Yi Ke Da Xue Xue Bao. 2023. PMID: 37202191 Free PMC article. Chinese.
-
N6-Methyladenosine Methyltransferase Component KIAA1429 Is a Potential Target of Cancer Therapy.Biomolecules. 2024 Oct 17;14(10):1319. doi: 10.3390/biom14101319. Biomolecules. 2024. PMID: 39456252 Free PMC article. Review.
-
Dynamic changes in the proximitome of neutral sphingomyelinase-2 (nSMase2) in TNFα stimulated Jurkat cells.Front Immunol. 2024 Jul 9;15:1435701. doi: 10.3389/fimmu.2024.1435701. eCollection 2024. Front Immunol. 2024. PMID: 39044828 Free PMC article.
References
-
- Adam, F., Kauskot, A., Kurowska, M., Goudin, N., Munoz, I., Bordet, J.C., Huang, J.D., Bryckaert, M., Fischer, A., Borgel, D., de Saint Basile, G., Christophe, O.D., and Ménasché, G.. 2018. Kinesin-1 is a new actor involved in platelet secretion and thrombus stability. Arterioscler. Thromb. Vasc. Biol., 38: 1037–1051. - PubMed
-
- Ailion, M., Hannemann, M., Dalton, S., Pappas, A., Watanabe, S., Hegermann, J., Liu, Q., Han, H.F., Gu, M., Goulding, M.Q., Sasidharan, N., Schuske, K., Hullett, P., Eimer, S., and Jorgensen, E.M.. 2014. Two Rab2 interactors regulate dense-core vesicle maturation. Neuron, 82: 167–180. - PMC - PubMed
-
- Alzahofi, N., Welz, T., Robinson, C.L., Page, E.L., Briggs, D.A., Stainthorp, A.K., Reekes, J., Elbe, D.A., Straub, F., Kallemeijn, W.W., Tate, E.W., Goff, P.S., Sviderskaya, E.V., Cantero, M., Montoliu, L., Nedelec, F., Miles, A.K., Bailly, M., Kerkhoff, E., and Hume, A.N.. 2020. Rab27a co-ordinates actin-dependent transport by controlling organelle-associated motors and track assembly proteins. Nat. Commun., 11: 3495. - PMC - PubMed

