Posterodorsal Medial Amygdala Regulation of Female Social Behavior: GABA versus Glutamate Projections
- PMID: 34470806
- PMCID: PMC8528505
- DOI: 10.1523/JNEUROSCI.1103-21.2021
Posterodorsal Medial Amygdala Regulation of Female Social Behavior: GABA versus Glutamate Projections
Abstract
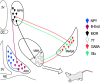
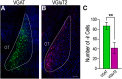
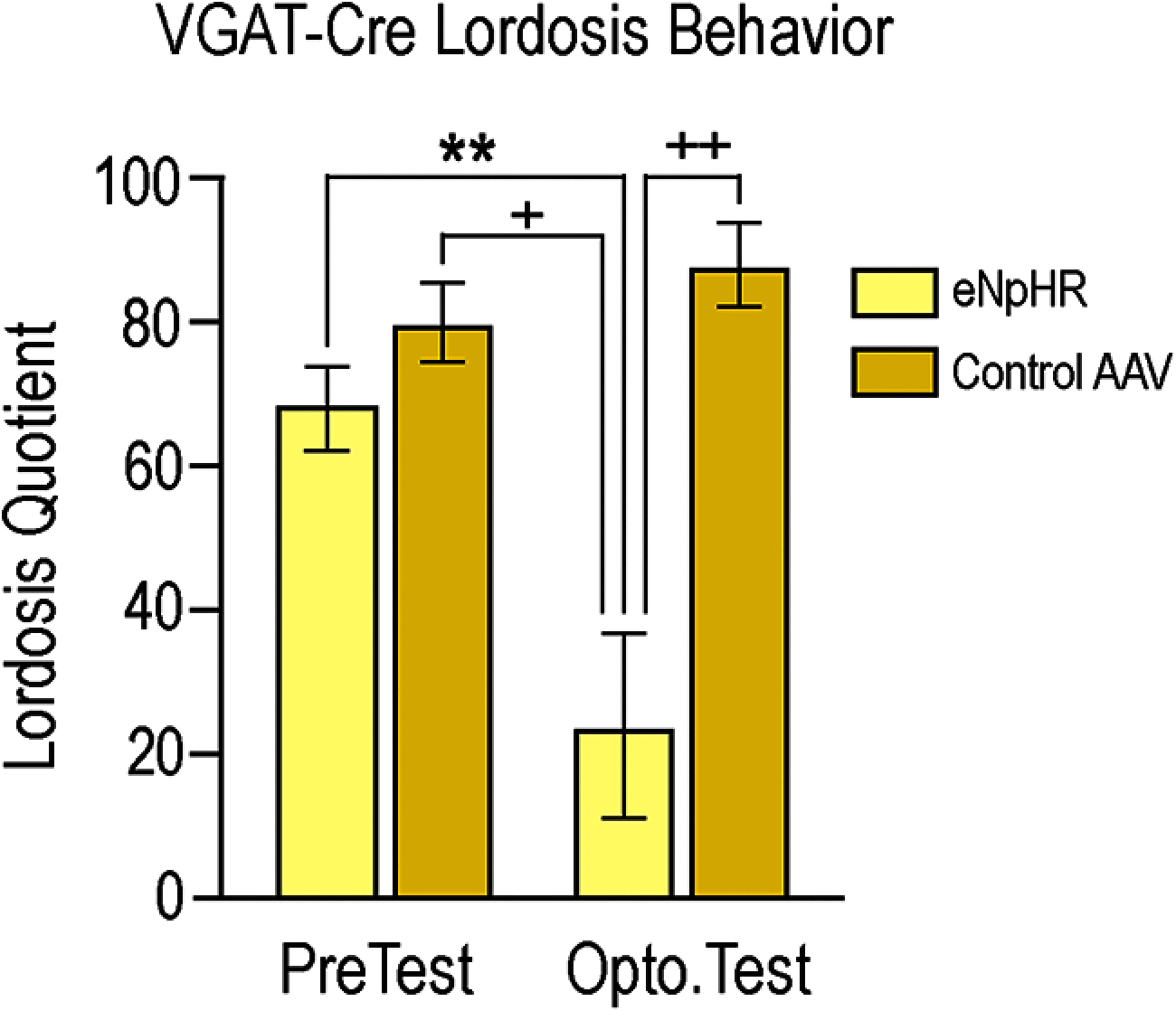
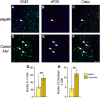
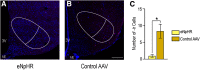
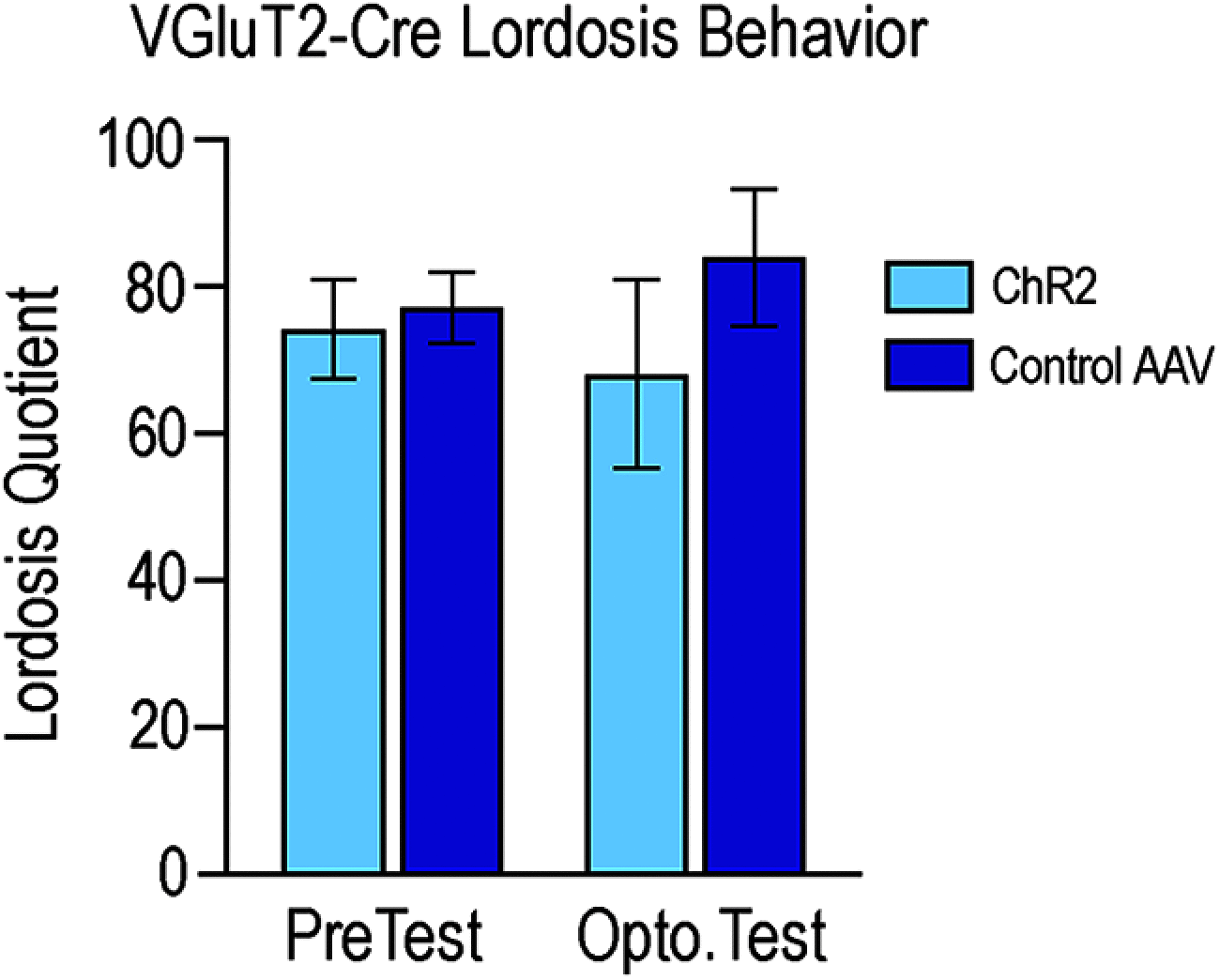
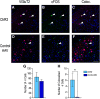
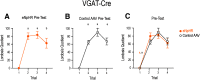
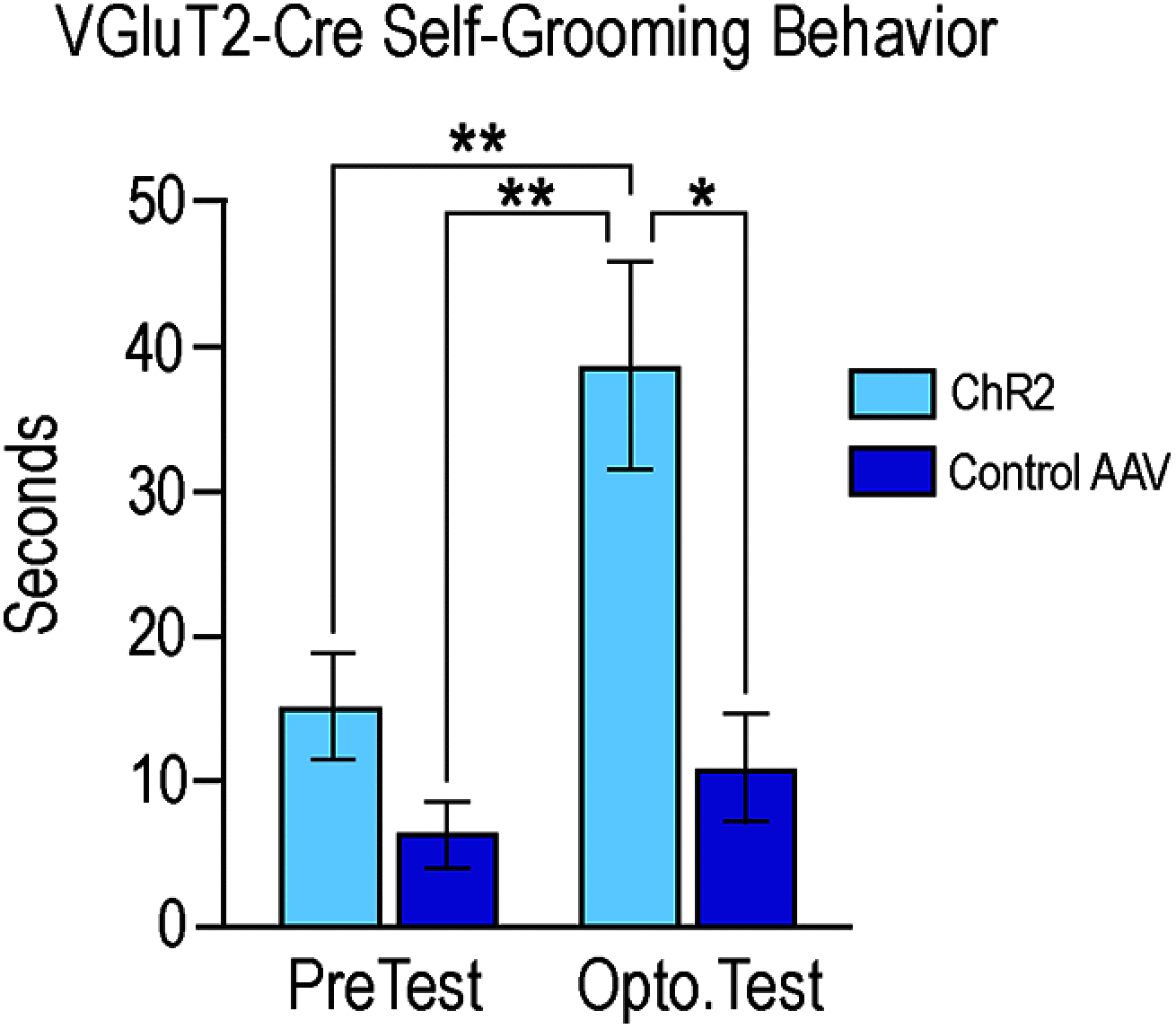
Social behaviors, including reproductive behaviors, often display sexual dimorphism. Lordosis, the measure of female sexual receptivity, is one of the most apparent sexually dimorphic reproductive behaviors. Lordosis is regulated by estrogen and progesterone (P4) acting within a hypothalamic-limbic circuit, consisting of the arcuate, medial preoptic, and ventromedial nuclei of the hypothalamus. Social cues are integrated into the circuit through the amygdala. The posterodorsal part of the medial amygdala (MeApd) is involved in sexually dimorphic social and reproductive behaviors, and sends projections to hypothalamic neuroendocrine regions. GABA from the MeApd appears to facilitate social behaviors, while glutamate may play the opposite role. To test these hypotheses, adult female vesicular GABA transporter (VGAT)-Cre and vesicular glutamate transporter 2 (VGluT2)-Cre mice were transfected with halorhodopsin (eNpHR)-expressing or channelrhodopsin-expressing adeno-associated viruses (AAVs), respectively, in the MeApd. The lordosis quotient (LQ) was measured following either photoinhibition of VGAT or photoexcitation of VGluT2 neurons, and brains were assessed for c-Fos immunohistochemistry (IHC). Photoinhibition of VGAT neurons in the MeApd decreased LQ, and decreased c-Fos expression within VGAT neurons, within the MeApd as a whole, and within the ventrolateral part of the ventromedial nucleus (VMHvl). Photoexcitation of VGluT2 neurons did not affect LQ, but did increase time spent self-grooming, and increased c-Fos expression within VGluT2 neurons in the MeApd. Neither condition altered c-Fos expression in the medial preoptic nucleus (MPN) or the arcuate nucleus (ARH). These data support a role for MeApd GABA in the facilitation of lordosis. Glutamate from the MeApd does not appear to be directly involved in the lordosis circuit, but appears to direct behavior away from social interactions.SIGNIFICANCE STATEMENT Lordosis, the measure of female sexual receptivity, is a sexually dimorphic behavior regulated within a hypothalamic-limbic circuit. Social cues are integrated through the amygdala, and the posterodorsal part of the medial amygdala (MeApd) is involved in sexually dimorphic social and reproductive behaviors. Photoinhibition of GABAergic neurons in the MeApd inhibited lordosis, while photoactivation of glutamate neurons had no effect on lordosis, but increased self-grooming. These data support a role for MeApd GABA in the facilitation of social behaviors and MeApd glutamate projections in anti-social interactions.
Copyright © 2021 Johnson et al.
Figures

Similar articles
-
Monosynaptic Inputs to Ventral Tegmental Area Glutamate and GABA Co-transmitting Neurons.J Neurosci. 2024 Nov 13;44(46):e2184232024. doi: 10.1523/JNEUROSCI.2184-23.2024. J Neurosci. 2024. PMID: 39327007
-
Optogenetic Activation of β-Endorphin Terminals in the Medial Preoptic Nucleus Regulates Female Sexual Receptivity.eNeuro. 2020 Jan 24;7(1):ENEURO.0315-19.2019. doi: 10.1523/ENEURO.0315-19.2019. Print 2020 Jan/Feb. eNeuro. 2020. PMID: 31941660 Free PMC article.
-
GABA and glutamate in mating-activated cells in the preoptic area and medial amygdala of male gerbils.J Comp Neurol. 2003 May 5;459(3):290-300. doi: 10.1002/cne.10605. J Comp Neurol. 2003. PMID: 12655511
-
Sexual dimorphism in the vomeronasal pathway and sex differences in reproductive behaviors.Brain Res Brain Res Rev. 1993 Jan-Apr;18(1):51-74. doi: 10.1016/0165-0173(93)90007-m. Brain Res Brain Res Rev. 1993. PMID: 8467350 Review.
-
Hypothalamic control of innate social behaviors.Science. 2023 Oct 27;382(6669):399-404. doi: 10.1126/science.adh8489. Epub 2023 Oct 26. Science. 2023. PMID: 37883550 Free PMC article. Review.
Cited by
-
Neuronal network dynamics in the posterodorsal amygdala: shaping reproductive hormone pulsatility.J R Soc Interface. 2024 Aug;21(217):20240143. doi: 10.1098/rsif.2024.0143. Epub 2024 Aug 28. J R Soc Interface. 2024. PMID: 39193642 Free PMC article.
-
Sex differences in mouse infralimbic cortex projections to the nucleus accumbens shell.Biol Sex Differ. 2023 Dec 11;14(1):87. doi: 10.1186/s13293-023-00570-3. Biol Sex Differ. 2023. PMID: 38082417 Free PMC article.
-
Neural substrates for regulating self-grooming behavior in rodents.J Zhejiang Univ Sci B. 2024 Jul 9;25(10):841-856. doi: 10.1631/jzus.B2300562. J Zhejiang Univ Sci B. 2024. PMID: 39420521 Free PMC article. Review.
-
Current Perspectives on Kisspeptins Role in Behaviour.Front Endocrinol (Lausanne). 2022 Jun 9;13:928143. doi: 10.3389/fendo.2022.928143. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 35757400 Free PMC article. Review.
-
The cAMP Response Element- Binding Protein/Brain-Derived Neurotrophic Factor Pathway in Anterior Cingulate Cortex Regulates Neuropathic Pain and Anxiodepression Like Behaviors in Rats.Front Mol Neurosci. 2022 Mar 24;15:831151. doi: 10.3389/fnmol.2022.831151. eCollection 2022. Front Mol Neurosci. 2022. PMID: 35401106 Free PMC article.
References
-
- Beach FA (1948) Hormones and behavior. New York: Paul B Hoeber.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

