Combination of enamel matrix derivative and hyaluronic acid inhibits lipopolysaccharide-induced inflammatory response on human epithelial and bone cells
- PMID: 34460002
- PMCID: PMC8816768
- DOI: 10.1007/s00784-021-04152-8
Combination of enamel matrix derivative and hyaluronic acid inhibits lipopolysaccharide-induced inflammatory response on human epithelial and bone cells
Abstract
Objectives: The aim of this study was to evaluate the in vitro effect of enamel matrix derivative (EMD) and hyaluronic acid (HA) and their synergistic combination on lipopolysaccharides (LPS)-induced inflammation in human keratinocytes and osteoblasts.
Material and methods: Cells were challenged with LPS (1 μg/ml) and cultured in the following treatment groups with EMD (30 mg/ml) and HA (30 mg/ml): LPS, EMD, HA, EMD + HA, EMD + LPS, HA + LPS, and EMD + HA + LPS. Cell viability, inflammatory cytokine expression, and cell migration were determined using colorimetric assay, quantitative real-time polymerase chain reaction (qPCR), and scratch wound healing assay, respectively.
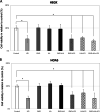
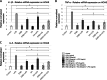
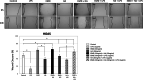
Results: Cell viability was decreased when exposed to LPS compared to the controls. Overall, LPS treatment expressed upregulation on inflammatory cytokine tumor necrosis factor alpha (TNF-α), interleukin 1 beta (IL-1β), and interleukin 6 (IL-6). EMD and HA reduced up to 3.0-fold the cytokine expression caused by LPS (p < 0.05). EMD and HA statistically induced higher migration in osteoblasts and keratinocytes, respectively. Migration was impaired by LPS, whereas it significantly increased after addition of EMD and HA.
Conclusions: EMD and HA are advantageous biomaterials that individually generate strong directional migratory keratinocyte and osteoblast response. Their combination also enhances cell viability, and anti-inflammatory and migratory abilities to promote healing specially under LPS inflammatory stimulus. Future in vivo and animal research is necessary to further characterize the effect of EMD and HA on periodontal regeneration.
Clinical relevance: The use of EMD in conjunction with HA resulted in a reduction of inflammation and improvement of tissue healing at wound sites. Both biomaterials combined may potentially improve the effectiveness of bone regeneration in periodontal bone defects, pointing to the potential clinical relevance of both materials in regenerative periodontal surgery.
Keywords: Bone regeneration; Cell viability; Enamel matrix derivative; Hyaluronic acid; Oral wound healing; Pro-inflammatory cytokines.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
Periodontal regeneration with a combination of enamel matrix proteins and autogenous bone grafting.J Periodontol. 2003 Sep;74(9):1269-81. doi: 10.1902/jop.2003.74.9.1269. J Periodontol. 2003. PMID: 14584859
-
Enamel matrix derivative exhibits anti-inflammatory properties in monocytes.J Periodontol. 2008 Mar;79(3):535-40. doi: 10.1902/jop.2008.070311. J Periodontol. 2008. PMID: 18315437
-
Anti-inflammatory properties of enamel matrix derivative in human blood.J Periodontal Res. 2006 Jun;41(3):208-13. doi: 10.1111/j.1600-0765.2005.00863.x. J Periodontal Res. 2006. PMID: 16677290
-
Enamel matrix derivative (Emdogain) for periodontal tissue regeneration in intrabony defects. A Cochrane systematic review.Eur J Oral Implantol. 2009 Winter;2(4):247-66. Eur J Oral Implantol. 2009. PMID: 20467602 Review.
-
The use of enamel matrix derivative in the treatment of periodontal defects: a literature review and meta-analysis.Crit Rev Oral Biol Med. 2004 Nov 1;15(6):382-402. doi: 10.1177/154411130401500605. Crit Rev Oral Biol Med. 2004. PMID: 15574680 Review.
Cited by
-
Assessment of Changes in the Oral Microbiome That Occur in Dogs with Periodontal Disease.Vet Sci. 2021 Nov 27;8(12):291. doi: 10.3390/vetsci8120291. Vet Sci. 2021. PMID: 34941818 Free PMC article.
-
TGF-β Signalling Mediates the Anti-Inflammatory Activity of Enamel Matrix Derivative In Vitro.Int J Mol Sci. 2022 Aug 29;23(17):9778. doi: 10.3390/ijms23179778. Int J Mol Sci. 2022. PMID: 36077174 Free PMC article.
-
Enamel matrix derivative in the treatment of tooth replantation: from a biological basis to clinical application.Ann Med. 2024 Dec;56(1):2424452. doi: 10.1080/07853890.2024.2424452. Epub 2024 Nov 9. Ann Med. 2024. PMID: 39520135 Free PMC article.
-
Enamel Matrix Derivative Decreases Pyroptosis-Related Genes in Macrophages.Int J Mol Sci. 2022 May 3;23(9):5078. doi: 10.3390/ijms23095078. Int J Mol Sci. 2022. PMID: 35563469 Free PMC article.
-
Efficiency of Hyaluronic Acid in Infrabony Defects: A Systematic Review of Human Clinical Trials.Medicina (Kaunas). 2022 Apr 23;58(5):580. doi: 10.3390/medicina58050580. Medicina (Kaunas). 2022. PMID: 35629997 Free PMC article. Review.
References
-
- Wikesjö UME, Selvig KA. Periodontal wound healing and regeneration. Periodontol 2000. 1999;19:21–39. - PubMed
-
- Bosshardt DD. Biological mediators and periodontal regeneration: a review of enamel matrix proteins at the cellular and molecular levels. J Clin Periodontol. 2008;35:87–105. - PubMed
-
- Trombelli L, Farina R. Clinical outcomes with bioactive agents alone or in combination with grafting or guided tissue regeneration. J Clin Periodontol. 2008;35:117–135. - PubMed
-
- Lee J, Stavropoulos A, Susin C, Wikesjö UME. Periodontal regeneration: focus on growth and differentiation factors. Dent Clin N Am. 2010;54:93–111. - PubMed
-
- Giannobile WV. Potential role of growth factors and differentiation factors in periodontal regeneration. Am Acad Periodontol J Periodontol. 1996;67:545–553. - PubMed

