Tools for functional dissection of site-specific O-GlcNAcylation
- PMID: 34458751
- PMCID: PMC8386111
- DOI: 10.1039/d0cb00052c
Tools for functional dissection of site-specific O-GlcNAcylation
Abstract
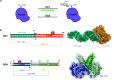
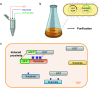
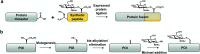
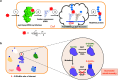
Protein O-GlcNAcylation is an abundant post-translational modification of intracellular proteins with the monosaccharide N-acetylglucosamine covalently tethered to serines and threonines. Modification of proteins with O-GlcNAc is required for metazoan embryo development and maintains cellular homeostasis through effects on transcription, signalling and stress response. While disruption of O-GlcNAc homeostasis can have detrimental impact on cell physiology and cause various diseases, little is known about the functions of individual O-GlcNAc sites. Most of the sites are modified sub-stoichiometrically which is a major challenge to the dissection of O-GlcNAc function. Here, we discuss the application, advantages and limitations of the currently available tools and technologies utilised to dissect the function of O-GlcNAc on individual proteins and sites in vitro and in vivo. Additionally, we provide a perspective on future developments required to decipher the protein- and site-specific roles of this essential sugar modification.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts of interest to declare.
Figures

Similar articles
-
Feedback Regulation of O-GlcNAc Transferase through Translation Control to Maintain Intracellular O-GlcNAc Homeostasis.Int J Mol Sci. 2021 Mar 27;22(7):3463. doi: 10.3390/ijms22073463. Int J Mol Sci. 2021. PMID: 33801653 Free PMC article.
-
Targeted Protein O-GlcNAcylation Using Bifunctional Small Molecules.J Am Chem Soc. 2024 Apr 10;146(14):9779-9789. doi: 10.1021/jacs.3c14380. Epub 2024 Apr 1. J Am Chem Soc. 2024. PMID: 38561350 Free PMC article.
-
Chemical biology tools to interrogate the roles of O-GlcNAc in immunity.Front Immunol. 2023 Jan 26;13:1089824. doi: 10.3389/fimmu.2022.1089824. eCollection 2022. Front Immunol. 2023. PMID: 36776401 Free PMC article. Review.
-
O-GlcNAcylation site mapping by (azide-alkyne) click chemistry and mass spectrometry following intensive fractionation of skeletal muscle cells proteins.J Proteomics. 2018 Aug 30;186:83-97. doi: 10.1016/j.jprot.2018.07.005. Epub 2018 Jul 26. J Proteomics. 2018. PMID: 30016717
-
Analytical and Biochemical Perspectives of Protein O-GlcNAcylation.Chem Rev. 2021 Feb 10;121(3):1513-1581. doi: 10.1021/acs.chemrev.0c00884. Epub 2021 Jan 8. Chem Rev. 2021. PMID: 33416322 Review.
Cited by
-
O-GlcNAc Engineering on a Target Protein in Cells with Nanobody-OGT and Nanobody-splitOGA.Curr Protoc. 2021 May;1(5):e117. doi: 10.1002/cpz1.117. Curr Protoc. 2021. PMID: 33950562 Free PMC article.
-
The O-GlcNAc Modification of Recombinant Tau Protein and Characterization of the O-GlcNAc Pattern for Functional Study.Methods Mol Biol. 2024;2754:237-269. doi: 10.1007/978-1-0716-3629-9_14. Methods Mol Biol. 2024. PMID: 38512671
-
Chemistry-Assisted Proteomic Profiling of O-GlcNAcylation.Front Chem. 2021 Jun 25;9:702260. doi: 10.3389/fchem.2021.702260. eCollection 2021. Front Chem. 2021. PMID: 34249870 Free PMC article. Review.
-
Target protein deglycosylation in living cells by a nanobody-fused split O-GlcNAcase.Nat Chem Biol. 2021 May;17(5):593-600. doi: 10.1038/s41589-021-00757-y. Epub 2021 Mar 8. Nat Chem Biol. 2021. PMID: 33686291 Free PMC article.
-
Tools for mammalian glycoscience research.Cell. 2022 Jul 21;185(15):2657-2677. doi: 10.1016/j.cell.2022.06.016. Epub 2022 Jul 8. Cell. 2022. PMID: 35809571 Free PMC article. Review.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

