Comparable Infection Level and Tropism of Measles Virus and Canine Distemper Virus in Organotypic Brain Slice Cultures Obtained from Natural Host Species
- PMID: 34452447
- PMCID: PMC8402773
- DOI: 10.3390/v13081582
Comparable Infection Level and Tropism of Measles Virus and Canine Distemper Virus in Organotypic Brain Slice Cultures Obtained from Natural Host Species
Abstract
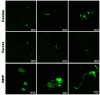
Measles virus (MV) and canine distemper virus (CDV) are closely related members of the family Paramyxoviridae, genus Morbillivirus. MV infection of humans and non-human primates (NHPs) results in a self-limiting disease, which rarely involves central nervous system (CNS) complications. In contrast, infection of carnivores with CDV usually results in severe disease, in which CNS complications are common and the case-fatality rate is high. To compare the neurovirulence and neurotropism of MV and CDV, we established a short-term organotypic brain slice culture system of the olfactory bulb, hippocampus, or cortex obtained from NHPs, dogs, and ferrets. Slices were inoculated ex vivo with wild-type-based recombinant CDV or MV expressing a fluorescent reporter protein. The infection level of both morbilliviruses was determined at different times post-infection. We observed equivalent infection levels and identified microglia as main target cells in CDV-inoculated carnivore and MV-inoculated NHP brain tissue slices. Neurons were also susceptible to MV infection in NHP brain slice cultures. Our findings suggest that MV and CDV have comparable neurotropism and intrinsic capacity to infect CNS-resident cells of their natural host species.
Keywords: canine distemper virus; central nervous system; measles virus; morbillivirus; organotypic brain slice culture; pathogenesis; tropism.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Measles vaccination of nonhuman primates provides partial protection against infection with canine distemper virus.J Virol. 2014 Apr;88(8):4423-33. doi: 10.1128/JVI.03676-13. Epub 2014 Feb 5. J Virol. 2014. PMID: 24501402 Free PMC article.
-
Rational attenuation of canine distemper virus (CDV) to develop a morbillivirus animal model that mimics measles in humans.J Virol. 2024 Mar 19;98(3):e0185023. doi: 10.1128/jvi.01850-23. Epub 2024 Feb 28. J Virol. 2024. PMID: 38415596 Free PMC article.
-
Morbillivirus Experimental Animal Models: Measles Virus Pathogenesis Insights from Canine Distemper Virus.Viruses. 2016 Oct 11;8(10):274. doi: 10.3390/v8100274. Viruses. 2016. PMID: 27727184 Free PMC article. Review.
-
Infection of ferrets with wild type-based recombinant canine distemper virus overwhelms the immune system and causes fatal systemic disease.mSphere. 2023 Aug 24;8(4):e0008223. doi: 10.1128/msphere.00082-23. Epub 2023 Jun 28. mSphere. 2023. PMID: 37377421 Free PMC article.
-
Tropism and molecular pathogenesis of canine distemper virus.Virol J. 2019 Mar 7;16(1):30. doi: 10.1186/s12985-019-1136-6. Virol J. 2019. PMID: 30845967 Free PMC article. Review.
Cited by
-
A highly potent human neutralizing antibody prevents vertical transmission of Rift Valley fever virus in a rat model.Nat Commun. 2023 Jul 26;14(1):4507. doi: 10.1038/s41467-023-40187-z. Nat Commun. 2023. PMID: 37495594 Free PMC article.
References
-
- Nikolin V.M., Olarte-Castillo X.A., Osterrieder N., Hofer H., Dubovi E., Mazzoni C.J., Brunner E., Goller K.V., Fyumagwa R.D., Moehlman P.D., et al. Canine distemper virus in the Serengeti ecosystem: Molecular adaptation to different carnivore species. Mol. Ecol. 2017;26:2111–2130. doi: 10.1111/mec.13902. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

