Development, Characterization and In Vivo Pharmacokinetic Assessment of Rectal Suppositories Containing Combination Antiretroviral Drugs for HIV Prevention
- PMID: 34452070
- PMCID: PMC8401959
- DOI: 10.3390/pharmaceutics13081110
Development, Characterization and In Vivo Pharmacokinetic Assessment of Rectal Suppositories Containing Combination Antiretroviral Drugs for HIV Prevention
Abstract
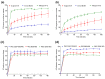
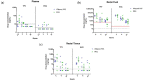
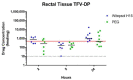
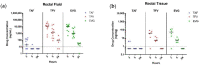
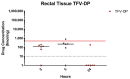
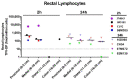
Receptive anal intercourse (RAI) contributes significantly to HIV acquisition underscoring the need to develop HIV prevention options for populations engaging in RAI practices. We explored the feasibility of formulating rectal suppositories with potent antiviral drugs for on-demand use. A fixed-dose combination of tenofovir (TFV) and elvitegravir (EVG) (40 mg each) was co-formulated in six different suppository bases (three fat- and three water-soluble). Fat-soluble witepsol H15 and water-soluble polyethylene glycol (PEG) based suppositories demonstrated favorable in vitro release and were advanced to assess in vivo pharmacokinetics following rectal administration in macaques. In vivo drug release profiles were similar for both suppository bases. Median concentrations of TFV and EVG detected in rectal fluids at 2 h were 1- and 2-logs higher than the in vitro IC50, respectively; TFV-diphosphate levels in rectal tissues met or exceeded those associated with high efficacy against rectal simian HIV (SHIV) exposure in macaques. Leveraging on these findings, a PEG-based suppository with a lower dose combination of tenofovir alafenamide (TAF) and EVG (8 mg each) was developed and found to achieve similar rectal drug exposures in macaques. This study establishes the utility of rectal suppositories as a promising on-demand strategy for HIV PrEP and supports their clinical development.
Keywords: HIV prevention; elvitegravir (EVG); non-human primate (NHP); pharmacokinetics (PK); pre-exposure prophylaxis (PrEP); prodrug; rectal microbicide (RM); rectal suppository; tenofovir alafenamide fumarate (TAF); tenofovir diphosphate (TFV-DP).
Conflict of interest statement
W.H. is named on patents and patent applications by the US government on methods of HIV prevention by chemoprophylaxis. All other authors declare no conflict of interest.
Figures
Similar articles
-
Pharmacokinetics and efficacy of topical inserts containing tenofovir alafenamide fumarate and elvitegravir administered rectally in macaques.EBioMedicine. 2022 Dec;86:104338. doi: 10.1016/j.ebiom.2022.104338. Epub 2022 Nov 5. EBioMedicine. 2022. PMID: 36343572 Free PMC article.
-
A Phase 1 Clinical Trial to Assess the Safety and Pharmacokinetics of a Tenofovir Alafenamide/Elvitegravir Insert Administered Rectally for HIV Prevention.J Infect Dis. 2024 Sep 23;230(3):696-705. doi: 10.1093/infdis/jiae211. J Infect Dis. 2024. PMID: 38655842 Clinical Trial.
-
Weekly Oral Tenofovir Alafenamide Protects Macaques from Vaginal and Rectal Simian HIV Infection.Pharmaceutics. 2024 Mar 11;16(3):384. doi: 10.3390/pharmaceutics16030384. Pharmaceutics. 2024. PMID: 38543278 Free PMC article.
-
Tenofovir alafenamide (TAF) as the successor of tenofovir disoproxil fumarate (TDF).Biochem Pharmacol. 2016 Nov 1;119:1-7. doi: 10.1016/j.bcp.2016.04.015. Epub 2016 Apr 29. Biochem Pharmacol. 2016. PMID: 27133890 Review.
-
Role of tenofovir alafenamide (TAF) in the treatment and prophylaxis of HIV and HBV infections.Biochem Pharmacol. 2018 Jul;153:2-11. doi: 10.1016/j.bcp.2017.11.023. Epub 2017 Dec 7. Biochem Pharmacol. 2018. PMID: 29225131 Review.
References
-
- Centers for Disease Control and Prevention HIV Surveillance Report, 2018 (Updated), Volume 31. [(accessed on 19 April 2021)];2020 May; Available online: http://www.cdc.gov/hiv/library/reports/hiv-surveillance.html.
LinkOut - more resources
Full Text Sources
Miscellaneous

