Design, synthesis, and evaluation of novel racecadotril-tetrazole-amino acid derivatives as new potent analgesic agents
- PMID: 34447443
- PMCID: PMC8356715
- DOI: 10.4103/1735-5362.319573
Design, synthesis, and evaluation of novel racecadotril-tetrazole-amino acid derivatives as new potent analgesic agents
Abstract
Background and purpose: Although pain is one of the most common symptoms of diseases, it is often mismanaged due to limited access to painkillers and ineffectiveness, unacceptable side effects, or the possibility of abuse. However, an alternative approach to existing analgesics is to indirectly increase endogenous pain relief pathways by neprilysin (an enkephalinase) inhibitors. This enzyme breaks down and inactivates enkephalin, dynorphin, endorphins, and their derivatives.
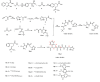
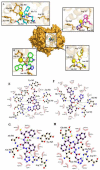
Experimental approach: In this project, a new series of racecadotril-tetrazole-amino acid derivatives 15a-l was synthesized and characterized on the basis of IR, 1H and 13C NMR, mass spectrometry, and elemental analysis. The antinociceptive activity of synthesized compounds was assessed by a hot plate, tail-flick, and formalin assays in mice. Docking was used to identify the possible interactions between neprilysin and synthesized compounds. 15a-l was synthesized and characterized on the basis of IR, 1H and 13C NMR, mass spectrometry, and elemental analysis. The antinociceptive activity of synthesized compounds was assessed by a hot plate, tail-flick, and formalin assays in mice. Docking was used to identify the possible interactions between neprilysin and synthesized compounds.
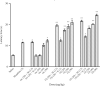
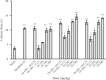
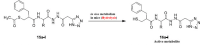
Findings/results: Most of the synthesized compounds showed moderate to good analgesic effects in hot plat and tail-flick test in comparison to morphine and racecadotril. Compounds 15l and 15j were the most potent compounds. The synergistic analgesic effect of compounds 15l and 15j with morphine and the antagonistic effect of naloxone on the activity of these compounds confirm that the analgesic effect of compounds 15l and 15j could be mediated through the opioidergic system. The negative and high binding energy of docking simulation of the most potent compounds in the catalytic site of neprilysin was also in good agreement with the inhibitory activity of test compounds.
Conclusion and implications: Racecadotril-tetrazole-amino acid derivatives, as potential antinociceptive agents, demonstrated moderate to good antinociceptive activities comparable with morphine and higher than racecadotril.
Keywords: Antinociceptive activity; Enkephalinase; Molecular docking simulation; Racecadotril; Tetrazole; Thiorphan.
Copyright: © 2021 Research in Pharmaceutical Sciences.
Conflict of interest statement
The authors declared no conflict of interest in this study.
Figures


Similar articles
-
Synergistic combinations of the dual enkephalinase inhibitor PL265 given orally with various analgesic compounds acting on different targets, in a murine model of cancer-induced bone pain.Scand J Pain. 2017 Jan;14:25-38. doi: 10.1016/j.sjpain.2016.09.011. Epub 2016 Nov 1. Scand J Pain. 2017. PMID: 28850427
-
Relationship between enkephalinase inhibition of thiorphan in vivo and its analgesic activity.J Pharmacobiodyn. 1985 Sep;8(9):701-10. doi: 10.1248/bpb1978.8.701. J Pharmacobiodyn. 1985. PMID: 3910797
-
New carboxyalkyl inhibitors of brain enkephalinase: synthesis, biological activity, and analgesic properties.J Med Chem. 1983 Jan;26(1):60-5. doi: 10.1021/jm00355a013. J Med Chem. 1983. PMID: 6298420
-
Indirect-acting strategy of opioid action instead of direct receptor activation: dual-acting enkephalinase inhibitors (DENKIs).J Clin Pharm Ther. 2018 Aug;43(4):443-449. doi: 10.1111/jcpt.12687. Epub 2018 May 2. J Clin Pharm Ther. 2018. PMID: 29722031 Review.
-
[Enkephalinase inhibitors and molecular study of the differences between active sites of enkephalinase and angiotensin-converting enzyme].J Pharmacol. 1985;16 Suppl 1:5-31. J Pharmacol. 1985. PMID: 2993752 Review. French.
Cited by
-
Synthesis of oxamide-hydrazone hybrid derivatives as potential anticancer agents.Res Pharm Sci. 2022 Dec 24;18(1):24-38. doi: 10.4103/1735-5362.363593. eCollection 2023 Feb. Res Pharm Sci. 2022. PMID: 36846733 Free PMC article.
-
Discovery of novel inhibitors of ghrelin O-acyltransferase enzyme: an in-silico approach.Res Pharm Sci. 2022 Sep 8;17(5):540-557. doi: 10.4103/1735-5362.355212. eCollection 2022 Oct. Res Pharm Sci. 2022. PMID: 36386482 Free PMC article.
References
-
- Bovill JG. Mechanisms of actions of opioids and non-steroidal anti-inflammatory drugs. Eur J Anaesthesiol Suppl. 1997;15:9–15. DOI: 10.1097/00003643-199705001-00003. - PubMed
-
- Liles JH, Flecknell PA. The use of non-steroidal anti-inflammatory drugs for the relief of pain in laboratory rodents and rabbits. Lab Anim. 1992;26(4):241–255. DOI: 10.1258/002367792780745706. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
