Alpha-1 Antitrypsin Augmentation Inhibits Proteolysis of Neutrophil Membrane Voltage-Gated Proton Channel-1 in Alpha-1 Deficient Individuals
- PMID: 34441020
- PMCID: PMC8398194
- DOI: 10.3390/medicina57080814
Alpha-1 Antitrypsin Augmentation Inhibits Proteolysis of Neutrophil Membrane Voltage-Gated Proton Channel-1 in Alpha-1 Deficient Individuals
Abstract
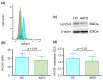
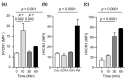
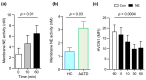
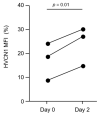
Background and Objectives: Alpha-1 antitrypsin is a serine protease inhibitor that demonstrates an array of immunomodulatory functions. Individuals with the genetic condition of alpha-1 antitrypsin deficiency (AATD) are at increased risk of early onset emphysematous lung disease. This lung disease is partly driven by neutrophil mediated lung destruction in an environment of low AAT. As peripheral neutrophil hyper-responsiveness in AATD leads to excessive degranulation and increased migration to the airways, we examined the expression of the membrane voltage-gated proton channel-1 (HVCN1), which is integrally linked to neutrophil function. The objectives of this study were to evaluate altered HVCN1 in AATD neutrophils, serine protease-dependent degradation of HVCN1, and to investigate the ability of serum AAT to control HVCN1 expression. Materials and Methods: Circulating neutrophils were purified from AATD patients (n = 20), AATD patients receiving AAT augmentation therapy (n = 3) and healthy controls (n = 20). HVCN1 neutrophil expression was assessed by flow cytometry and Western blot analysis. Neutrophil membrane bound elastase was measured by fluorescence resonance energy transfer. Results: In this study we demonstrated that HVCN1 protein is under-expressed in AATD neutrophils (p = 0.02), suggesting a link between reduced HVCN1 expression and AAT deficiency. We have demonstrated that HVCN1 undergoes significant proteolytic degradation in activated neutrophils (p < 0.0001), primarily due to neutrophil elastase activity (p = 0.0004). In addition, the treatment of AATD individuals with AAT augmentation therapy increased neutrophil plasma membrane HVCN1 expression (p = 0.01). Conclusions: Our results demonstrate reduced levels of HVCN1 in peripheral blood neutrophils that may influence the neutrophil-dominated immune response in the AATD airways and highlights the role of antiprotease treatment and specifically AAT augmentation therapy in protecting neutrophil membrane expression of HVCN1.
Keywords: alpha-1 antitrypsin; elastase; neutrophils; voltage-gated proton channel-1.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
α1 Antitrypsin therapy modulates the neutrophil membrane proteome and secretome.Eur Respir J. 2020 Apr 30;55(4):1901678. doi: 10.1183/13993003.01678-2019. Print 2020 Apr. Eur Respir J. 2020. PMID: 32060059
-
The BLT1 Inhibitory Function of α-1 Antitrypsin Augmentation Therapy Disrupts Leukotriene B4 Neutrophil Signaling.J Immunol. 2015 Oct 15;195(8):3628-41. doi: 10.4049/jimmunol.1500038. Epub 2015 Sep 14. J Immunol. 2015. PMID: 26371243
-
Alpha-1 antitrypsin augmentation therapy corrects accelerated neutrophil apoptosis in deficient individuals.J Immunol. 2014 Oct 15;193(8):3978-91. doi: 10.4049/jimmunol.1400132. Epub 2014 Sep 12. J Immunol. 2014. PMID: 25217166
-
The Role of Neutrophils in Alpha-1 Antitrypsin Deficiency.Ann Am Thorac Soc. 2016 Aug;13 Suppl 4:S297-304. doi: 10.1513/AnnalsATS.201509-634KV. Ann Am Thorac Soc. 2016. PMID: 27564664 Review.
-
Long-term clinical outcomes following treatment with alpha 1-proteinase inhibitor for COPD associated with alpha-1 antitrypsin deficiency: a look at the evidence.Respir Res. 2017 May 30;18(1):105. doi: 10.1186/s12931-017-0574-1. Respir Res. 2017. PMID: 28558837 Free PMC article. Review.
Cited by
-
C3d Elicits Neutrophil Degranulation and Decreases Endothelial Cell Migration, with Implications for Patients with Alpha-1 Antitrypsin Deficiency.Biomedicines. 2021 Dec 16;9(12):1925. doi: 10.3390/biomedicines9121925. Biomedicines. 2021. PMID: 34944741 Free PMC article.
-
Respiratory Immune Responses during Infection and Pollution Inhalation.Medicina (Kaunas). 2023 Jan 27;59(2):242. doi: 10.3390/medicina59020242. Medicina (Kaunas). 2023. PMID: 36837444 Free PMC article.
References
-
- Rotondo J.C., Oton-Gonzalez L., Selvatici R., Rizzo P., Pavasini R., Campo G.C., Lanzillotti C., Mazziotta C., De Mattei M., Tognon M., et al. SERPINA1 Gene Promoter Is Differentially Methylated in Peripheral Blood Mononuclear Cells of Pregnant Women. Front. Cell Dev. Biol. 2020;8:550543. doi: 10.3389/fcell.2020.550543. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

