Human Vascular Smooth Muscle Function and Oxidative Stress Induced by NADPH Oxidase with the Clinical Implications
- PMID: 34440716
- PMCID: PMC8393371
- DOI: 10.3390/cells10081947
Human Vascular Smooth Muscle Function and Oxidative Stress Induced by NADPH Oxidase with the Clinical Implications
Abstract
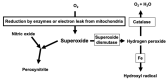
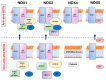
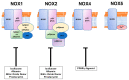
Among reactive oxygen species, superoxide mediates the critical vascular redox signaling, resulting in the regulation of the human cardiovascular system. The reduced form of nicotinamide adenine dinucleotide phosphate oxidase (NADPH oxidase, NOX) is the source of superoxide and relates to the crucial intracellular pathology and physiology of vascular smooth muscle cells, including contraction, proliferation, apoptosis, and inflammatory response. Human vascular smooth muscle cells express NOX1, 2, 4, and 5 in physiological and pathological conditions, and those enzymes play roles in most cardiovascular disorders caused by hypertension, diabetes, inflammation, and arteriosclerosis. Various physiologically active substances, including angiotensin II, stimulate NOX via the cytosolic subunits' translocation toward the vascular smooth muscle cell membrane. As we have shown, some pathological stimuli such as high glucose augment the enzymatic activity mediated by the phosphatidylinositol 3-kinase-Akt pathway, resulting in the membrane translocation of cytosolic subunits of NOXs. This review highlights and details the roles of human vascular smooth muscle NOXs in the pathophysiology and clinical aspects. The regulation of the enzyme expressed in the vascular smooth muscle cells may lead to the prevention and treatment of human cardiovascular diseases.
Keywords: NADPH oxidase; human vascular smooth muscle; oxidase stress.
Conflict of interest statement
Hiroyuki Kinoshita is a consult of IMI Co. Ltd., Koshigaya, Saitama, Japan. The funder had no role in the writing of the manuscript.
Figures
Similar articles
-
Synthetic smooth muscle cell phenotype is associated with increased nicotinamide adenine dinucleotide phosphate oxidase activity: effect on collagen secretion.J Vasc Surg. 2006 Feb;43(2):364-71. doi: 10.1016/j.jvs.2005.10.032. J Vasc Surg. 2006. PMID: 16476616
-
Osteoprotegerin regulates vascular function through syndecan-1 and NADPH oxidase-derived reactive oxygen species.Clin Sci (Lond). 2021 Oct 29;135(20):2429-2444. doi: 10.1042/CS20210643. Clin Sci (Lond). 2021. PMID: 34668009
-
NADPH oxidases and vascular remodeling in cardiovascular diseases.Pharmacol Res. 2016 Dec;114:110-120. doi: 10.1016/j.phrs.2016.10.015. Epub 2016 Oct 20. Pharmacol Res. 2016. PMID: 27773825 Review.
-
NADPH oxidase (NOX) 1 mediates cigarette smoke-induced superoxide generation in rat vascular smooth muscle cells.Toxicol In Vitro. 2017 Feb;38:49-58. doi: 10.1016/j.tiv.2016.10.013. Epub 2016 Nov 3. Toxicol In Vitro. 2017. PMID: 27816504
-
NADPH oxidases in vascular pathology.Antioxid Redox Signal. 2014 Jun 10;20(17):2794-814. doi: 10.1089/ars.2013.5607. Epub 2013 Nov 1. Antioxid Redox Signal. 2014. PMID: 24180474 Free PMC article. Review.
Cited by
-
Endothelial Dysfunction and Chronic Inflammation: The Cornerstones of Vascular Alterations in Age-Related Diseases.Int J Mol Sci. 2022 Dec 11;23(24):15722. doi: 10.3390/ijms232415722. Int J Mol Sci. 2022. PMID: 36555364 Free PMC article. Review.
-
NADPH Oxidases in Diastolic Dysfunction and Heart Failure with Preserved Ejection Fraction.Antioxidants (Basel). 2022 Sep 16;11(9):1822. doi: 10.3390/antiox11091822. Antioxidants (Basel). 2022. PMID: 36139898 Free PMC article. Review.
-
Chemical Constituents, In Vitro Antioxidant Activity and In Silico Study on NADPH Oxidase of Allium sativum L. (Garlic) Essential Oil.Antioxidants (Basel). 2021 Nov 20;10(11):1844. doi: 10.3390/antiox10111844. Antioxidants (Basel). 2021. PMID: 34829715 Free PMC article.
-
Ferroptosis due to Cystathionine γ Lyase/Hydrogen Sulfide Downregulation Under High Hydrostatic Pressure Exacerbates VSMC Dysfunction.Front Cell Dev Biol. 2022 Feb 3;10:829316. doi: 10.3389/fcell.2022.829316. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35186934 Free PMC article.
-
The role of oxidative stress in aortic dissection: a potential therapeutic target.Front Cardiovasc Med. 2024 Jul 12;11:1410477. doi: 10.3389/fcvm.2024.1410477. eCollection 2024. Front Cardiovasc Med. 2024. PMID: 39070552 Free PMC article. Review.
References
-
- Guzik T.J., Chen W., Gongora M.C., Guzik B., Lob H.E., Mangalat D., Hoch N., Dikalov S., Rudzinski P., Kapelak B., et al. Calcium-dependent NOX5 nicotinamide adenine dinucleotide phosphate oxidase contributes to vascular oxidative stress in human coronary artery disease. J. Am. Coll. Cardiol. 2008;52:1803–1809. doi: 10.1016/j.jacc.2008.07.063. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

