Risks and Function of Breast Cancer Susceptibility Alleles
- PMID: 34439109
- PMCID: PMC8393346
- DOI: 10.3390/cancers13163953
Risks and Function of Breast Cancer Susceptibility Alleles
Abstract
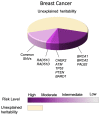
Family history remains one of the strongest risk factors for breast cancer. It is well established that women with a first-degree relative affected by breast cancer are twice as likely to develop the disease themselves. Twins studies indicate that this is most likely due to shared genetics rather than shared epidemiological/lifestyle risk factors. Linkage and targeted sequencing studies have shown that rare high- and moderate-penetrance germline variants in genes involved in the DNA damage response (DDR) including BRCA1, BRCA2, PALB2, ATM, and TP53 are responsible for a proportion of breast cancer cases. However, breast cancer is a heterogeneous disease, and there is now strong evidence that different risk alleles can predispose to different subtypes of breast cancer. Here, we review the associations between the different genes and subtype-specificity of breast cancer based on the most comprehensive genetic studies published. Genome-wide association studies (GWAS) have also been used to identify an additional hereditary component of breast cancer, and have identified hundreds of common, low-penetrance susceptibility alleles. The combination of these low penetrance risk variants, summed as a polygenic risk score (PRS), can identify individuals across the spectrum of disease risk. However, there remains a substantial bottleneck between the discovery of GWAS-risk variants and their contribution to tumorigenesis mainly because the majority of these variants map to the non-protein coding genome. A range of functional genomic approaches are needed to identify the causal risk variants and target susceptibility genes and establish their underlying role in disease biology. We discuss how the application of these multidisciplinary approaches to understand genetic risk for breast cancer can be used to identify individuals in the population that may benefit from clinical interventions including screening for early detection and prevention, and treatment strategies to reduce breast cancer-related mortalities.
Keywords: GWAS; breast cancer risk; clinical genetic testing; functional genomics; subtype-specific risk.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Common breast cancer risk variants in the post-COGS era: a comprehensive review.Breast Cancer Res. 2013 Dec 20;15(6):212. doi: 10.1186/bcr3591. Breast Cancer Res. 2013. PMID: 24359602 Free PMC article. Review.
-
Genetic epidemiology of ovarian cancer and prospects for polygenic risk prediction.Gynecol Oncol. 2017 Dec;147(3):705-713. doi: 10.1016/j.ygyno.2017.10.001. Epub 2017 Oct 18. Gynecol Oncol. 2017. PMID: 29054568 Review.
-
Breast cancer genes: beyond BRCA1 and BRCA2.Front Biosci (Landmark Ed). 2013 Jun 1;18(4):1358-72. doi: 10.2741/4185. Front Biosci (Landmark Ed). 2013. PMID: 23747889 Review.
-
Screening for BRCA1, BRCA2, CHEK2, PALB2, BRIP1, RAD50, and CDH1 mutations in high-risk Finnish BRCA1/2-founder mutation-negative breast and/or ovarian cancer individuals.Breast Cancer Res. 2011 Feb 28;13(1):R20. doi: 10.1186/bcr2832. Breast Cancer Res. 2011. PMID: 21356067 Free PMC article.
-
Health influenced by genetics: A first comprehensive analysis of breast cancer high and moderate penetrance susceptibility genes in the Tunisian population.PLoS One. 2022 Mar 25;17(3):e0265638. doi: 10.1371/journal.pone.0265638. eCollection 2022. PLoS One. 2022. PMID: 35333900 Free PMC article.
Cited by
-
A Large Case-Control Study Performed in Spanish Population Suggests That RECQL5 Is the Only RECQ Helicase Involved in Breast Cancer Susceptibility.Cancers (Basel). 2022 Sep 28;14(19):4738. doi: 10.3390/cancers14194738. Cancers (Basel). 2022. PMID: 36230663 Free PMC article.
-
Breast Cancer Screening and Prophylactic Mastectomy for High-Risk Women in Romania.Medicina (Kaunas). 2024 Mar 30;60(4):570. doi: 10.3390/medicina60040570. Medicina (Kaunas). 2024. PMID: 38674216 Free PMC article. Review.
-
The mortalities of female-specific cancers in China and other countries with distinct socioeconomic statuses: A longitudinal study.J Adv Res. 2023 Jul;49:127-139. doi: 10.1016/j.jare.2022.09.002. Epub 2022 Sep 18. J Adv Res. 2023. PMID: 36130684 Free PMC article.
-
Influence of selection on the probability of fixation at a locus with multiple alleles.BMC Genomics. 2024 Aug 30;25(1):819. doi: 10.1186/s12864-024-10733-0. BMC Genomics. 2024. PMID: 39215209 Free PMC article.
-
Impact on breast cancer susceptibility and clinicopathological traits of common genetic polymorphisms in TP53, MDM2 and ATM genes in Sardinian women.Oncol Lett. 2022 Aug 8;24(4):331. doi: 10.3892/ol.2022.13451. eCollection 2022 Oct. Oncol Lett. 2022. PMID: 36039053 Free PMC article.
References
-
- Cancer Facts & Figures 2021. [(accessed on 6 July 2021)]; Available online: https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts....
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

