In Vivo Models and In Vitro Assays for the Assessment of Pertussis Toxin Activity
- PMID: 34437436
- PMCID: PMC8402560
- DOI: 10.3390/toxins13080565
In Vivo Models and In Vitro Assays for the Assessment of Pertussis Toxin Activity
Abstract
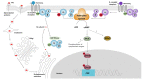
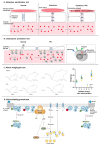
One of the main virulence factors produced by Bordetella pertussis is pertussis toxin (PTx) which, in its inactivated form, is the major component of all marketed acellular pertussis vaccines. PTx ADP ribosylates Gαi proteins, thereby affecting the inhibition of adenylate cyclases and resulting in the accumulation of cAMP. Apart from this classical model, PTx also activates some receptors and can affect various ADP ribosylation- and adenylate cyclase-independent signalling pathways. Due to its potent ADP-ribosylation properties, PTx has been used in many research areas. Initially the research primarily focussed on the in vivo effects of the toxin, including histamine sensitization, insulin secretion and leukocytosis. Nowadays, PTx is also used in toxicology research, cell signalling, research involving the blood-brain barrier, and testing of neutralizing antibodies. However, the most important area of use is testing of acellular pertussis vaccines for the presence of residual PTx. In vivo models and in vitro assays for PTx often reflect one of the toxin's properties or details of its mechanism. Here, the established and novel in vivo and in vitro methods used to evaluate PTx are reviewed, their mechanisms, characteristics and limitations are described, and their application for regulatory and research purposes are considered.
Keywords: acellular pertussis vaccines; in vitro assays; in vivo models; pertussis toxin.
Conflict of interest statement
The author declares no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures

Similar articles
-
Characterization of the carbohydrate binding and ADP-ribosyltransferase activities of chemically detoxified pertussis toxins.Vaccine. 2013 Jun 24;31(29):2988-93. doi: 10.1016/j.vaccine.2013.04.060. Epub 2013 May 9. Vaccine. 2013. PMID: 23664992
-
ADP-ribosylation activity in pertussis vaccines and its relationship to the in vivo histamine-sensitisation test.Vaccine. 2007 Apr 30;25(17):3311-8. doi: 10.1016/j.vaccine.2007.01.009. Epub 2007 Jan 19. Vaccine. 2007. PMID: 17287049
-
Reporter cell lines for detection of pertussis toxin in acellular pertussis vaccines as a functional animal-free alternative to the in vivo histamine sensitization test.Vaccine. 2017 Feb 22;35(8):1152-1160. doi: 10.1016/j.vaccine.2017.01.011. Epub 2017 Jan 24. Vaccine. 2017. PMID: 28129894
-
Assays for Determining Pertussis Toxin Activity in Acellular Pertussis Vaccines.Toxins (Basel). 2019 Jul 17;11(7):417. doi: 10.3390/toxins11070417. Toxins (Basel). 2019. PMID: 31319496 Free PMC article. Review.
-
The inhibitory G protein G(i) identified as pertussis toxin-catalyzed ADP-ribosylation.Biol Pharm Bull. 2012;35(12):2103-11. doi: 10.1248/bpb.b212024. Biol Pharm Bull. 2012. PMID: 23207763 Review.
Cited by
-
Safety assessments of recombinant DTaP vaccines developed in South Korea.Clin Exp Vaccine Res. 2024 Apr;13(2):155-165. doi: 10.7774/cevr.2024.13.2.155. Epub 2024 Apr 30. Clin Exp Vaccine Res. 2024. PMID: 38752005 Free PMC article.
-
Non-primate animal models for pertussis: back to the drawing board?Appl Microbiol Biotechnol. 2022 Feb;106(4):1383-1398. doi: 10.1007/s00253-022-11798-1. Epub 2022 Feb 1. Appl Microbiol Biotechnol. 2022. PMID: 35103810 Free PMC article. Review.
-
The CHO Cell Clustering Response to Pertussis Toxin: History of Its Discovery and Recent Developments in Its Use.Toxins (Basel). 2021 Nov 19;13(11):815. doi: 10.3390/toxins13110815. Toxins (Basel). 2021. PMID: 34822599 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

