Efficacy of Bamlanivimab/Etesevimab and Casirivimab/Imdevimab in Preventing Progression to Severe COVID-19 and Role of Variants of Concern
- PMID: 34435337
- PMCID: PMC8386337
- DOI: 10.1007/s40121-021-00525-4
Efficacy of Bamlanivimab/Etesevimab and Casirivimab/Imdevimab in Preventing Progression to Severe COVID-19 and Role of Variants of Concern
Abstract
Introduction: The aim of this study was to evaluate the risk of hospitalization or death in patients infected by SARS-CoV2 variants of concern (VOCs) receiving combinations of monoclonal antibodies (mAbs), bamlanivimab/etesevimab or casirivimab/imdevimab.
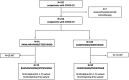
Methods: Observational prospective study conducted in two Italian hospitals (University Hospital of Pisa and San Donato Hospital, Arezzo) including consecutive outpatients with COVID-19 who received bamlanivimab/etesevimab or casirivimab/imdevimab from March 20th to May 10th 2021. All patients were at high risk of COVID-19 progression according to FDA/AIFA recommendations. Patients were divided into two study groups according to the infecting viral strain (VOCs): Alpha and Gamma group. The primary endpoint was a composite of hospitalization or death within 30 days from mAbs infusion. A Cox regression multivariate analysis was performed to identify factors associated with the primary outcome in the overall population.
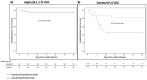
Results: The study included 165 patients: 105 were infected by the VOC Alpha and 43 by the VOC Gamma. In the Alpha group, no differences in the primary endpoint were observed between patients treated with bamlanivimab/etesevimab or casirivimab/imdevimab. Conversely, in the Gamma group, a higher proportion of patients treated with bamlanivimab/etesevimab met the primary endpoint compared to those receiving casirivimab/imdevimab (55% vs. 17.4%, p = 0.013). On multivariate Cox-regression analysis, the Gamma variant and days from symptoms onset to mAbs infusion were factors independently associated with higher risk of hospitalization or death, while casirivimab/imdevimab was protective (HR 0.33, 95% CI 0.13-0.83, p = 0.019).
Conclusions: In patients infected by the SARS-CoV-2 Gamma variant, bamlanivimab/etesevimab should be used with caution because of the high risk of disease progression.
Keywords: Bamlanivimab/etesevimab; COVID-19; Casirivimab/imdevimab; Monoclonal antibodies; SARS-CoV-2; Variants of concern.
© 2021. The Author(s).
Figures
Similar articles
-
Clinical efficacy of different monoclonal antibody regimens among non-hospitalised patients with mild to moderate COVID-19 at high risk for disease progression: a prospective cohort study.Eur J Clin Microbiol Infect Dis. 2022 Jul;41(7):1065-1076. doi: 10.1007/s10096-022-04464-x. Epub 2022 Jun 21. Eur J Clin Microbiol Infect Dis. 2022. PMID: 35727429 Free PMC article.
-
SARS-CoV-2-neutralising monoclonal antibodies for treatment of COVID-19.Cochrane Database Syst Rev. 2021 Sep 2;9(9):CD013825. doi: 10.1002/14651858.CD013825.pub2. Cochrane Database Syst Rev. 2021. PMID: 34473343 Free PMC article. Review.
-
Exploratory data on the clinical efficacy of monoclonal antibodies against SARS-CoV-2 Omicron variant of concern.Elife. 2022 Nov 22;11:e79639. doi: 10.7554/eLife.79639. Elife. 2022. PMID: 36413383 Free PMC article. Clinical Trial.
-
Curbing the Delta Surge: Clinical Outcomes After Treatment With Bamlanivimab-Etesevimab, Casirivimab-Imdevimab, or Sotrovimab for Mild to Moderate Coronavirus Disease 2019.Mayo Clin Proc. 2022 Sep;97(9):1641-1648. doi: 10.1016/j.mayocp.2022.06.015. Epub 2022 Jun 23. Mayo Clin Proc. 2022. PMID: 36058578 Free PMC article.
-
A Narrative Review of the Clinical Practicalities of Bamlanivimab and Etesevimab Antibody Therapies for SARS-CoV-2.Infect Dis Ther. 2021 Dec;10(4):1933-1947. doi: 10.1007/s40121-021-00515-6. Epub 2021 Aug 10. Infect Dis Ther. 2021. PMID: 34374951 Free PMC article. Review.
Cited by
-
Efficacy and Safety of Nirmatrelvir/Ritonavir, Molnupiravir, and Remdesivir in a Real-World Cohort of Outpatients with COVID-19 at High Risk of Progression: The PISA Outpatient Clinic Experience.Infect Dis Ther. 2023 Jan;12(1):257-271. doi: 10.1007/s40121-022-00729-2. Epub 2022 Nov 28. Infect Dis Ther. 2023. PMID: 36441485 Free PMC article.
-
Broadly neutralizing SARS-CoV-2 antibodies through epitope-based selection from convalescent patients.Nat Commun. 2023 Feb 8;14(1):687. doi: 10.1038/s41467-023-36295-5. Nat Commun. 2023. PMID: 36755042 Free PMC article.
-
Early Use of Remdesivir and Risk of Disease Progression in Hospitalized Patients With Mild to Moderate COVID-19.Clin Ther. 2022 Mar;44(3):364-373. doi: 10.1016/j.clinthera.2022.01.007. Epub 2022 Jan 17. Clin Ther. 2022. PMID: 35120742 Free PMC article.
-
Adrenomedullin Therapy in Moderate to Severe COVID-19.Biomedicines. 2022 Feb 24;10(3):533. doi: 10.3390/biomedicines10030533. Biomedicines. 2022. PMID: 35327335 Free PMC article. Review.
-
Monoclonal antibodies for the treatment of COVID-19 patients: An umbrella to overcome the storm?Int Immunopharmacol. 2021 Dec;101(Pt A):108200. doi: 10.1016/j.intimp.2021.108200. Epub 2021 Sep 28. Int Immunopharmacol. 2021. PMID: 34607231 Free PMC article. Review.
References
-
- Falcone M, Tiseo G, Barbieri G, Galfo V, Russo A, Virdis A, et al. Role of low-molecular-weight heparin in hospitalized patients with severe acute respiratory syndrome Coronavirus 2 pneumonia: a prospective observational study. Open Forum Infect Dis. 2020;7:ofaa563. doi: 10.1093/ofid/ofaa563. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

