mRNA vaccines for infectious diseases: principles, delivery and clinical translation
- PMID: 34433919
- PMCID: PMC8386155
- DOI: 10.1038/s41573-021-00283-5
mRNA vaccines for infectious diseases: principles, delivery and clinical translation
Erratum in
-
Author Correction: mRNA vaccines for infectious diseases: principles, delivery and clinical translation.Nat Rev Drug Discov. 2021 Nov;20(11):880. doi: 10.1038/s41573-021-00321-2. Nat Rev Drug Discov. 2021. PMID: 34548658 Free PMC article. No abstract available.
Abstract
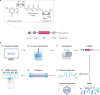
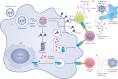
Over the past several decades, messenger RNA (mRNA) vaccines have progressed from a scepticism-inducing idea to clinical reality. In 2020, the COVID-19 pandemic catalysed the most rapid vaccine development in history, with mRNA vaccines at the forefront of those efforts. Although it is now clear that mRNA vaccines can rapidly and safely protect patients from infectious disease, additional research is required to optimize mRNA design, intracellular delivery and applications beyond SARS-CoV-2 prophylaxis. In this Review, we describe the technologies that underlie mRNA vaccines, with an emphasis on lipid nanoparticles and other non-viral delivery vehicles. We also overview the pipeline of mRNA vaccines against various infectious disease pathogens and discuss key questions for the future application of this breakthrough vaccine platform.
© 2021. Springer Nature Limited.
Conflict of interest statement
K.A.W. is an inventor on the following patents related to lipid nanoparticles: US Patent 8,450,298; 8,969,353; 9,556,110; 9,872,911; 9,227,917; 9,439,968; 10,189,802; and 10,844,028. K.A.W. is bound by confidential agreements that prevent her from disclosing related consulting relationships.
Figures


Similar articles
-
Advances in the design and delivery of RNA vaccines for infectious diseases.Adv Drug Deliv Rev. 2024 Oct;213:115419. doi: 10.1016/j.addr.2024.115419. Epub 2024 Aug 5. Adv Drug Deliv Rev. 2024. PMID: 39111358 Review.
-
Oral mRNA Vaccines Against Infectious Diseases- A Bacterial Perspective [Invited].Front Immunol. 2022 May 3;13:884862. doi: 10.3389/fimmu.2022.884862. eCollection 2022. Front Immunol. 2022. PMID: 35592330 Free PMC article. Review.
-
Engineering of the current nucleoside-modified mRNA-LNP vaccines against SARS-CoV-2.Biomed Pharmacother. 2021 Oct;142:111953. doi: 10.1016/j.biopha.2021.111953. Epub 2021 Jul 23. Biomed Pharmacother. 2021. PMID: 34343897 Free PMC article. Review.
-
The Rapid Development and Early Success of Covid 19 Vaccines Have Raised Hopes for Accelerating the Cancer Treatment Mechanism.Arch Razi Inst. 2021 Mar;76(1):1-6. doi: 10.22092/ari.2021.353761.1612. Epub 2021 Mar 1. Arch Razi Inst. 2021. PMID: 33818952 Free PMC article.
-
COVID-19 mRNA vaccines: Platforms and current developments.Mol Ther. 2022 May 4;30(5):1850-1868. doi: 10.1016/j.ymthe.2022.02.016. Epub 2022 Feb 19. Mol Ther. 2022. PMID: 35189345 Free PMC article. Review.
Cited by
-
A chimeric mRNA vaccine of S-RBD with HA conferring broad protection against influenza and COVID-19 variants.PLoS Pathog. 2024 Sep 20;20(9):e1012508. doi: 10.1371/journal.ppat.1012508. eCollection 2024 Sep. PLoS Pathog. 2024. PMID: 39303003 Free PMC article.
-
Herpes zoster mRNA vaccine induces superior vaccine immunity over licensed vaccine in mice and rhesus macaques.Emerg Microbes Infect. 2024 Dec;13(1):2309985. doi: 10.1080/22221751.2024.2309985. Epub 2024 Feb 11. Emerg Microbes Infect. 2024. PMID: 38258878 Free PMC article.
-
Optimized inhaled LNP formulation for enhanced treatment of idiopathic pulmonary fibrosis via mRNA-mediated antibody therapy.Nat Commun. 2024 Aug 10;15(1):6844. doi: 10.1038/s41467-024-51056-8. Nat Commun. 2024. PMID: 39122711 Free PMC article.
-
Template-dependent DNA ligation for the synthesis of modified oligonucleotides.Nat Commun. 2024 Sep 13;15(1):8009. doi: 10.1038/s41467-024-52141-8. Nat Commun. 2024. PMID: 39271668 Free PMC article.
-
Assessment of Human SARS CoV-2-Specific T-Cell Responses Elicited In Vitro by New Computationally Designed mRNA Immunogens (COVARNA).Vaccines (Basel). 2023 Dec 22;12(1):15. doi: 10.3390/vaccines12010015. Vaccines (Basel). 2023. PMID: 38250827 Free PMC article.
References
-
- Verbeke R, Lentacker I, De Smedt SC, Dewitte H. Three decades of messenger RNA vaccine development. Nano Today. 2019;28:100766. doi: 10.1016/j.nantod.2019.100766. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

