Osthole induces apoptosis of the HT-29 cells via endoplasmic reticulum stress and autophagy
- PMID: 34429766
- PMCID: PMC8371959
- DOI: 10.3892/ol.2021.12987
Osthole induces apoptosis of the HT-29 cells via endoplasmic reticulum stress and autophagy
Abstract
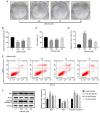
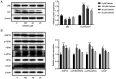
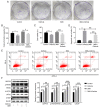
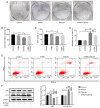
Endoplasmic reticulum stress (ERS) and autophagy are important pathways, which induce apoptosis of tumor cells. Osthole has been demonstrated to exert anticancer effects via the induction of apoptosis in several human colon cancer lines, but the mechanism underlying its involvement in the induction of ERS and autophagy in the human HT-29 colorectal cancer cell line remains unknown. The present study aimed to identify the possible signaling pathways involved in osthole-induced apoptosis of HT29 cells. Methodologically, colony formation and Cell Counting Kit-8 assays were used to assess cell proliferation and viability, respectively, while flow cytometry was performed to investigate apoptosis. Signaling pathways, including apoptosis, autophagy and ERS, were also investigated in the HT-29 cell line using western blot analysis. The results demonstrated that osthole inhibited cellular proliferation and viability in a dose-dependent manner. In addition, osthole induced the expression level of proteins associated with mitochondria-mediated cell apoptosis, autophagy and ERS. The association between autophagy and ERS in osthole-induced apoptosis in the HT-29 cell line was further clarified. Inhibiting cell autophagy with the inhibitor, 3-methyladenine, suppressed osthole-induced cell apoptosis and enhanced osthole-induced ERS. By contrast, alleviating ERS with the inhibitor, 4-phenylbutyric acid attenuated osthole-induced cell apoptosis and autophagy. In conclusion, osthole could significantly suppress the proliferation and viability of the HT-29 colorectal cancer cell line and induce cell apoptosis via autophagy and ERS. Furthermore, ERS may play a more important role in osthole-induced cell apoptosis.
Keywords: apoptosis; autophagy; colon cancer; endoplasmic reticulum stress; osthole.
Copyright: © Zhou et al.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Similar articles
-
Alleviation of endoplasmic reticulum stress protects against cisplatin-induced ovarian damage.Reprod Biol Endocrinol. 2018 Sep 3;16(1):85. doi: 10.1186/s12958-018-0404-4. Reprod Biol Endocrinol. 2018. PMID: 30176887 Free PMC article.
-
Cucurbitacin B induces apoptosis in colorectal cells through reactive oxygen species generation and endoplasmic reticulum stress pathways.Exp Ther Med. 2023 Sep 1;26(4):484. doi: 10.3892/etm.2023.12183. eCollection 2023 Oct. Exp Ther Med. 2023. PMID: 37753296 Free PMC article.
-
Overexpression of lncRNA Dancr inhibits apoptosis and enhances autophagy to protect cardiomyocytes from endoplasmic reticulum stress injury via sponging microRNA-6324.Mol Med Rep. 2021 Feb;23(2):116. doi: 10.3892/mmr.2020.11755. Epub 2020 Dec 10. Mol Med Rep. 2021. PMID: 33300079 Free PMC article.
-
Induction mechanisms of autophagy and endoplasmic reticulum stress in intestinal ischemia-reperfusion injury, inflammatory bowel disease, and colorectal cancer.Biomed Pharmacother. 2024 Jan;170:115984. doi: 10.1016/j.biopha.2023.115984. Epub 2023 Dec 8. Biomed Pharmacother. 2024. PMID: 38070244 Review.
-
The Road of Solid Tumor Survival: From Drug-Induced Endoplasmic Reticulum Stress to Drug Resistance.Front Mol Biosci. 2021 Apr 13;8:620514. doi: 10.3389/fmolb.2021.620514. eCollection 2021. Front Mol Biosci. 2021. PMID: 33928116 Free PMC article. Review.
Cited by
-
The dual effect of endoplasmic reticulum stress in digestive system tumors and intervention of Chinese botanical drug extracts: a review.Front Pharmacol. 2024 Feb 21;15:1339146. doi: 10.3389/fphar.2024.1339146. eCollection 2024. Front Pharmacol. 2024. PMID: 38449811 Free PMC article. Review.
-
Osthole: An up-to-date review of its anticancer potential and mechanisms of action.Front Pharmacol. 2022 Sep 7;13:945627. doi: 10.3389/fphar.2022.945627. eCollection 2022. Front Pharmacol. 2022. PMID: 36160431 Free PMC article. Review.
-
Osthole inhibits malignant phenotypes and induces ferroptosis in KRAS-mutant colorectal cancer cells via suppressing AMPK/Akt signaling.Cancer Chemother Pharmacol. 2023 Aug;92(2):119-134. doi: 10.1007/s00280-023-04549-0. Epub 2023 Jun 15. Cancer Chemother Pharmacol. 2023. PMID: 37318525
-
A review on the mechanisms underlying the antitumor effects of natural products by targeting the endoplasmic reticulum stress apoptosis pathway.Front Pharmacol. 2023 Nov 17;14:1293130. doi: 10.3389/fphar.2023.1293130. eCollection 2023. Front Pharmacol. 2023. PMID: 38044941 Free PMC article. Review.
-
TCM targets ferroptosis: potential treatments for cancer.Front Pharmacol. 2024 Apr 22;15:1360030. doi: 10.3389/fphar.2024.1360030. eCollection 2024. Front Pharmacol. 2024. PMID: 38738174 Free PMC article. Review.
References
-
- Bao Y, Meng X, Liu F, Wang F, Yang J, Wang H, Xie G. Protective effects of osthole against inflammation induced by lipopolysaccharide in BV2 cells. Mol Med Rep. 2018;17:4561–4566. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
