Antibiotic Resistance and Biofilm Production Capacity in Clostridioides difficile
- PMID: 34422678
- PMCID: PMC8371447
- DOI: 10.3389/fcimb.2021.683464
Antibiotic Resistance and Biofilm Production Capacity in Clostridioides difficile
Abstract
Background: Clostridioides difficile (C. difficile) is one of the primary pathogens responsible for infectious diarrhea. Antibiotic treatment failure, occurring in about 30% of patients, and elevated rates of antibiotic resistance pose a major challenge for therapy. Reinfection often occurs by isolates that produce biofilm, a protective barrier impermeable to antibiotics. We explored the association between antibiotic resistance (in planktonic form) and biofilm-production in 123 C. difficile clinical isolates.
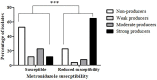
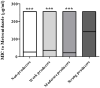
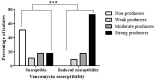
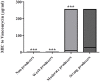
Results: Overall, 66 (53.6%) out of 123 isolates produced a biofilm, with most of them being either a strong (44%) or moderate (34.8%) biofilm producers. When compared to susceptible isolates, a statistically higher percentage of isolates with reduced susceptibility to metronidazole or vancomycin were biofilm producers (p < 0.0001, for both antibiotics). Biofilm production intensity was higher among tolerant isolates; 53.1% of the metronidazole-susceptible isolates were not able to produce biofilms, and only 12.5% were strong biofilm-producers. In contrast, 63% of the isolates with reduced susceptibility had a strong biofilm-production capability, while 22.2% were non-producers. Among the vancomycin-susceptible isolates, 51% were unable to produce biofilms, while all the isolates with reduced vancomycin susceptibility were biofilm-producers. Additionally, strong biofilm production capacity was more common among the isolates with reduced vancomycin susceptibility, compared to susceptible isolates (72.7% vs. 18.8%, respectively). The distribution of biofilm capacity groups was statistically different between different Sequence-types (ST) strains (p =0.001). For example, while most of ST2 (66.7%), ST13 (60%), ST42 (80%) isolates were non-producers, most (75%) ST6 isolates were moderate producers and most of ST104 (57.1%) were strong producers.
Conclusions: Our results suggest an association between reduced antibiotic susceptibility and biofilm production capacity. This finding reinforces the importance of antibiotic susceptibility testing, mainly in recurrence infections that may be induced by a strain that is both antibiotic tolerant and biofilm producer. Better adjustment of treatment in such cases may reduce recurrences rates and complications. The link of biofilm production and ST should be further validated; if ST can indicate on isolate virulence, then in the future, when strain typing methods will be more available to laboratories, ST determination may aid in indecision between supportive vs. aggressive treatment.
Keywords: C. difficile; biofilm production; metronidazole; recurrence; reduced antibiotic susceptibility; vancomycin.
Copyright © 2021 Rahmoun, Azrad and Peretz.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
High sporulation and overexpression of virulence factors in biofilms and reduced susceptibility to vancomycin and linezolid in recurrent Clostridium [Clostridioides] difficile infection isolates.PLoS One. 2019 Jul 31;14(7):e0220671. doi: 10.1371/journal.pone.0220671. eCollection 2019. PLoS One. 2019. PMID: 31365590 Free PMC article.
-
Virulence factors, antibiotic susceptibility and sequence type distribution of hospital-associated Clostridioides difficile isolates in Israel, 2020-2022.Sci Rep. 2024 Sep 4;14(1):20607. doi: 10.1038/s41598-024-71492-2. Sci Rep. 2024. PMID: 39232075 Free PMC article.
-
Reassessment of Clostridium difficile susceptibility to metronidazole and vancomycin.Antimicrob Agents Chemother. 2002 Jun;46(6):1647-50. doi: 10.1128/AAC.46.6.1647-1650.2002. Antimicrob Agents Chemother. 2002. PMID: 12019070 Free PMC article.
-
Clostridioides difficile resistance to antibiotics, including post-COVID-19 data.Expert Rev Clin Pharmacol. 2023 Jul-Dec;16(10):925-938. doi: 10.1080/17512433.2023.2252331. Epub 2023 Aug 29. Expert Rev Clin Pharmacol. 2023. PMID: 37642560 Review.
-
Biofilm regulation in Clostridioides difficile: Novel systems linked to hypervirulence.PLoS Pathog. 2021 Sep 9;17(9):e1009817. doi: 10.1371/journal.ppat.1009817. eCollection 2021 Sep. PLoS Pathog. 2021. PMID: 34499698 Free PMC article. Review.
Cited by
-
Impact of Subinhibitory Concentrations of Metronidazole on Morphology, Motility, Biofilm Formation and Colonization of Clostridioides difficile.Antibiotics (Basel). 2022 May 5;11(5):624. doi: 10.3390/antibiotics11050624. Antibiotics (Basel). 2022. PMID: 35625268 Free PMC article.
-
Research Progress on the Combination of Quorum-Sensing Inhibitors and Antibiotics against Bacterial Resistance.Molecules. 2024 Apr 8;29(7):1674. doi: 10.3390/molecules29071674. Molecules. 2024. PMID: 38611953 Free PMC article. Review.
-
Risk Factors, Diagnosis, and Management of Clostridioides difficile Infection in Patients with Inflammatory Bowel Disease.Microorganisms. 2022 Jun 29;10(7):1315. doi: 10.3390/microorganisms10071315. Microorganisms. 2022. PMID: 35889034 Free PMC article. Review.
-
The effect of different C. difficile MLST strains on viability and activity of macrophages.Heliyon. 2023 Feb 17;9(3):e13846. doi: 10.1016/j.heliyon.2023.e13846. eCollection 2023 Mar. Heliyon. 2023. PMID: 36873553 Free PMC article.
-
Intestinal biofilms: pathophysiological relevance, host defense, and therapeutic opportunities.Clin Microbiol Rev. 2024 Sep 12;37(3):e0013323. doi: 10.1128/cmr.00133-23. Epub 2024 Jul 12. Clin Microbiol Rev. 2024. PMID: 38995034 Review.
References
-
- Arciola C. R., Campoccia D., Gamberini S., Donati M. E., Pirini V., Visai L., et al. . (2005). Antibiotic Resistance in Exopolysaccharide-Forming Staphylococcus Epidermidis Clinical Isolates From Orthopaedic Implant Infections. Biomaterials 26, 6530–6535. 10.1016/j.biomaterials.2005.04.031 - DOI - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources

