TMEM120A contains a specific coenzyme A-binding site and might not mediate poking- or stretch-induced channel activities in cells
- PMID: 34409941
- PMCID: PMC8480983
- DOI: 10.7554/eLife.71474
TMEM120A contains a specific coenzyme A-binding site and might not mediate poking- or stretch-induced channel activities in cells
Abstract
TMEM120A, a member of the transmembrane protein 120 (TMEM120) family, has a pivotal function in adipocyte differentiation and metabolism, and may also contribute to sensing mechanical pain by functioning as an ion channel named TACAN. Here we report that expression of TMEM120A is not sufficient in mediating poking- or stretch-induced currents in cells and have solved cryo-electron microscopy (cryo-EM) structures of human TMEM120A (HsTMEM120A) in complex with an endogenous metabolic cofactor (coenzyme A, CoASH) and in the apo form. HsTMEM120A forms a symmetrical homodimer with each monomer containing an amino-terminal coiled-coil motif followed by a transmembrane domain with six membrane-spanning helices. Within the transmembrane domain, a CoASH molecule is hosted in a deep cavity and forms specific interactions with nearby amino acid residues. Mutation of a central tryptophan residue involved in binding CoASH dramatically reduced the binding affinity of HsTMEM120A with CoASH. HsTMEM120A exhibits distinct conformations at the states with or without CoASH bound. Our results suggest that TMEM120A may have alternative functional roles potentially involved in CoASH transport, sensing, or metabolism.
Keywords: TMEM120; coenzyme A; cryo-EM structure; human; ion channel; mechanosensation; membrane protein; molecular biophysics; mouse; neuroscience; structural biology.
© 2021, Rong et al.
Conflict of interest statement
YR, JJ, YG, JG, DS, WL, MZ, YZ, BX, ZL No competing interests declared
Figures



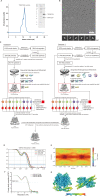








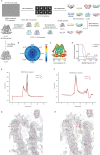

Comment in
-
Pain or gain?Elife. 2021 Sep 29;10:e73378. doi: 10.7554/eLife.73378. Elife. 2021. PMID: 34583807 Free PMC article.
Similar articles
-
TMEM120A/TACAN: A putative regulator of ion channels, mechanosensation, and lipid metabolism.Channels (Austin). 2023 Dec;17(1):2237306. doi: 10.1080/19336950.2023.2237306. Channels (Austin). 2023. PMID: 37523628 Free PMC article. Review.
-
TMEM120A is a coenzyme A-binding membrane protein with structural similarities to ELOVL fatty acid elongase.Elife. 2021 Aug 10;10:e71220. doi: 10.7554/eLife.71220. Elife. 2021. PMID: 34374645 Free PMC article.
-
Analysis of the mechanosensor channel functionality of TACAN.Elife. 2021 Aug 10;10:e71188. doi: 10.7554/eLife.71188. Elife. 2021. PMID: 34374644 Free PMC article.
-
Pain or gain?Elife. 2021 Sep 29;10:e73378. doi: 10.7554/eLife.73378. Elife. 2021. PMID: 34583807 Free PMC article.
-
Emerging issues of connexin channels: biophysics fills the gap.Q Rev Biophys. 2001 Aug;34(3):325-472. doi: 10.1017/s0033583501003705. Q Rev Biophys. 2001. PMID: 11838236 Review.
Cited by
-
Force From Filaments: The Role of the Cytoskeleton and Extracellular Matrix in the Gating of Mechanosensitive Channels.Front Cell Dev Biol. 2022 May 2;10:886048. doi: 10.3389/fcell.2022.886048. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35586339 Free PMC article. Review.
-
TMEM120A/TACAN: A putative regulator of ion channels, mechanosensation, and lipid metabolism.Channels (Austin). 2023 Dec;17(1):2237306. doi: 10.1080/19336950.2023.2237306. Channels (Austin). 2023. PMID: 37523628 Free PMC article. Review.
-
Phosphatidic acid is an endogenous negative regulator of PIEZO2 channels and mechanical sensitivity.bioRxiv [Preprint]. 2024 Mar 2:2024.03.01.582964. doi: 10.1101/2024.03.01.582964. bioRxiv. 2024. Update in: Nat Commun. 2024 Aug 15;15(1):7020. doi: 10.1038/s41467-024-51181-4 PMID: 38464030 Free PMC article. Updated. Preprint.
-
TMEM120A-mediated regulation of chemotherapy sensitivity in colorectal cancer cells.Cancer Chemother Pharmacol. 2024 Jan;93(1):11-22. doi: 10.1007/s00280-023-04594-9. Epub 2023 Sep 20. Cancer Chemother Pharmacol. 2024. PMID: 37728615
-
Genomic loci mispositioning in Tmem120a knockout mice yields latent lipodystrophy.Nat Commun. 2022 Jan 13;13(1):321. doi: 10.1038/s41467-021-27869-2. Nat Commun. 2022. PMID: 35027552 Free PMC article.
References
-
- Adams PD, Afonine PV, Bunkóczi G, Chen VB, Davis IW, Echols N, Headd JJ, Hung LW, Kapral GJ, Grosse-Kunstleve RW, McCoy AJ, Moriarty NW, Oeffner R, Read RJ, Richardson DC, Richardson JS, Terwilliger TC, Zwart PH. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallographica Section D Biological Crystallography. 2010;66:213–221. doi: 10.1107/S0907444909052925. - DOI - PMC - PubMed
-
- Beaulieu-Laroche L, Christin M, Donoghue A, Agosti F, Yousefpour N, Petitjean H, Davidova A, Stanton C, Khan U, Dietz C, Faure E, Fatima T, MacPherson A, Mouchbahani-Constance S, Bisson DG, Haglund L, Ouellet JA, Stone LS, Samson J, Smith MJ, Ask K, Ribeiro-da-Silva A, Blunck R, Poole K, Bourinet E, Sharif-Naeini R. TACAN Is an ion channel involved in sensing mechanical Pain. Cell. 2020;180:956–967. doi: 10.1016/j.cell.2020.01.033. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

