Real-world implementation of sequential targeted therapies for EGFR-mutated lung cancer
- PMID: 34408792
- PMCID: PMC8366107
- DOI: 10.1177/1758835921996509
Real-world implementation of sequential targeted therapies for EGFR-mutated lung cancer
Abstract
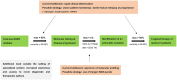
Background: Epidermal growth factor receptor-mutated (EGFR+) non-small-cell lung cancer (NSCLC) patients failing tyrosine kinase inhibitors (TKI) can benefit from next-line targeted therapies, but implementation is challenging.
Methods: EGFR+ NSCLC patients treated with first/second-generation (1G/2G) TKI at our institution with a last follow-up after osimertinib approval (February 2016), were analyzed retrospectively, and the results compared with published data under osimertinib.
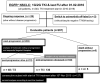
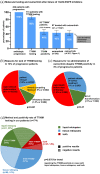
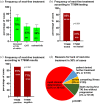
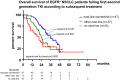
Results: A total of 207 patients received erlotinib (37%), gefitinib (16%) or afatinib (47%). The median age was 66 years, with a predominance of female (70%), never/light-smokers (69%). T790M testing was performed in 174/202 progressive cases (86%), positive in 93/174 (53%), and followed by osimertinib in 87/93 (94%). Among the 135 deceased patients, 94 (70%) received subsequent systemic treatment (43% chemotherapy, 39% osimertinib), while 30% died without, either before (4%) or after progression, due to rapid clinical deterioration (22%), patient refusal of further therapy (2%), or severe competing illness (2%). Lack of subsequent treatment was significantly (4.5x, p < 0.001) associated with lack of T790M testing, whose most frequent cause (in approximately 50% of cases) was also rapid clinical decline. Among the 127 consecutive patients with failure of 1G/2G TKI started after November 2015, 47 (37%) received osimertinib, with a median overall survival of 36 months versus 24 and 21 months for patients with alternative and no subsequent therapies (p = 0.003).
Conclusion: Osimertinib after 1G/2G TKI failure prolongs survival, but approximately 15% and 30% of patients forego molecular retesting and subsequent treatment, respectively, mainly due to rapid clinical deterioration. This is an important remediable obstacle to sequential TKI treatment for EGFR+ NSCLC. It pertains also to other actionable resistance mechanisms emerging under 1G/2G inhibitors or osimertinib, whose rate for lack of next-line therapy is similar (approximately 35% in the FLAURA/AURA3 trials), and highlights the need for closer monitoring alongside broader profiling of TKI-treated EGFR+ NSCLC in the future.
Keywords: EGFR T790M mutation; EGFR+ NSCLC; overall survival; rebiopsy; second line; tyrosine kinase inhibitor.
© The Author(s), 2021.
Conflict of interest statement
Conflict of interest statement: FB reports personal fees from Novartis, MSD, Chugai Pharma, Roche, and AstraZeneca and research grants from AstraZeneca, BMS, and Roche. ALV reports personal fees from AstraZeneca. DK reports personal fees from AstraZeneca, personal fees from Bristol-Myers Squibb GmbH, personal fees from Pfizer Pharma GmbH, outside the submitted work. FJH reports advisory board fees and honoraria from Lilly, Roche, AstraZeneca, Novartis, Boehringer, Chiesi, Teva, Pulmonx BTG, and Olympus as well as research funding from Lilly, Roche, AstraZeneca, Novartis, Boehringer, Chiesi, and Teva. CPH reports consultation, lecture and other fees from Novartis, Basilea, Bayer, Grifols, Boehringer, Pierre Fabre, Covidien, Siemens, Chiesi, Intermune, MEDA Pharma, Bracco, Pfizer, MSD, Roche, Lilly, AstraZeneca, Schering-Plough, Essex, Gilead, MeVis, Fresenius, and Astellas as well as ownership of GSK stocks TM reports research funding from Roche and patents with Roche. JRF reports advisory board honoraria from Boehringer, Roche, Celgene, and AstraZeneca. PS reports advisory board honoraria from Pfizer, Roche, Novartis, and AstraZeneca as well as speaker’s honoraria and research funding from Roche, AstraZeneca, and Novartis. AS reports advisory board honoraria and/or speaker fees: Astra Zeneca, Bayer, Eli Lilly, Roche, BMS, Illumina, MSD, Novartis, Pfizer, Seattle Genetics, Takeda, and Thermo Fisher, and research grants from BMS, Bayer, and Chugai. MT reports advisory board honoraria from Novartis, Lilly, BMS, MSD, Roche, Celgene, Takeda, AbbVie, Boehringer, speaker’s honoraria from Lilly, MSD, Takeda, research funding from AstraZeneca, BMS, Celgene, Novartis, Roche and travel grants from BMS, MSD, Novartis, Boehringer. PC reports lecture/advisory board fees from AstraZeneca, Boehringer, Chugai, Novartis, Pfizer, Roche and Takeda, as well as research funding from AstraZeneca, Novartis, Roche, and Takeda.
Figures
Similar articles
-
Osimertinib in the treatment of non-small-cell lung cancer: design, development and place in therapy.Lung Cancer (Auckl). 2017 Aug 18;8:109-125. doi: 10.2147/LCTT.S119644. eCollection 2017. Lung Cancer (Auckl). 2017. PMID: 28860885 Free PMC article. Review.
-
Treatment patterns, testing practices, and outcomes in the pre-FLAURA era for patients with EGFR mutation-positive advanced NSCLC: a retrospective chart review (REFLECT).Ther Adv Med Oncol. 2021 Nov 29;13:17588359211059874. doi: 10.1177/17588359211059874. eCollection 2021. Ther Adv Med Oncol. 2021. PMID: 35173817 Free PMC article.
-
Observational Study of Treatment Patterns in Patients with Epidermal Growth Factor Receptor (EGFR) Mutation-Positive Non-Small Cell Lung Cancer After First-Line EGFR-Tyrosine Kinase Inhibitors.Adv Ther. 2020 Feb;37(2):946-954. doi: 10.1007/s12325-020-01221-4. Epub 2020 Jan 18. Adv Ther. 2020. PMID: 31955357
-
Treatment Patterns, Testing Practices, and Outcomes in Patients with EGFR Mutation-Positive Advanced Non-Small-Cell Lung Cancer in Poland: A Descriptive Analysis of National, Multicenter, Real-World Data from the REFLECT Study.Cancers (Basel). 2023 Mar 3;15(5):1581. doi: 10.3390/cancers15051581. Cancers (Basel). 2023. PMID: 36900371 Free PMC article.
-
Ideal sequencing in Stage IV epidermal growth factor receptor mutant Non-Small-Cell Lung Cancer.Indian J Cancer. 2022 Mar;59(Supplement):S80-S89. doi: 10.4103/ijc.IJC_50_21. Indian J Cancer. 2022. PMID: 35343193 Review.
Cited by
-
Sequential treatment in advanced epidermal growth factor receptor-mutated lung adenocarcinoma patients receiving first-line bevacizumab combined with 1st/2nd-generation EGFR-tyrosine kinase inhibitors.Front Oncol. 2023 Oct 3;13:1249106. doi: 10.3389/fonc.2023.1249106. eCollection 2023. Front Oncol. 2023. PMID: 37854677 Free PMC article.
-
Feasibility and Challenges for Sequential Treatments in ALK-Rearranged Non-Small-Cell Lung Cancer.Front Oncol. 2021 Apr 20;11:670483. doi: 10.3389/fonc.2021.670483. eCollection 2021. Front Oncol. 2021. PMID: 33959513 Free PMC article.
-
Differential prognostic value of tumor and plasma T790M mutations in EGFR TKI-treated advanced NSCLC.Ther Adv Med Oncol. 2024 Jan 19;16:17588359231222604. doi: 10.1177/17588359231222604. eCollection 2024. Ther Adv Med Oncol. 2024. PMID: 38249338 Free PMC article.
-
APE1 shRNA-loaded cancer stem cell-derived extracellular vesicles reverse Erlotinib resistance in non-small cell lung cancer via the IL-6/STAT3 signalling.Clin Transl Med. 2022 May;12(5):e876. doi: 10.1002/ctm2.876. Clin Transl Med. 2022. PMID: 35605028 Free PMC article.
-
Activity of afatinib in patients with NSCLC harboring novel uncommon EGFR mutations with or without co-mutations: a case report.Front Oncol. 2024 May 6;14:1347742. doi: 10.3389/fonc.2024.1347742. eCollection 2024. Front Oncol. 2024. PMID: 38769948 Free PMC article.
References
-
- Ramalingam SS, Vansteenkiste J, Planchard D, et al.. Overall survival with osimertinib in untreated, EGFR-mutated advanced NSCLC. N Engl J Med 2020; 382: 41–50. - PubMed
-
- Mok TS, Cheng Y, Zhou X, et al.. Improvement in overall survival in a randomized study that compared dacomitinib with gefitinib in patients with advanced non-small-cell lung cancer and EGFR-activating mutations. J Clin Oncol 2018; 36: 2244–2250. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

