Vorinostat in autophagic cell death: A critical insight into autophagy-mediated, -associated and -dependent cell death for cancer prevention
- PMID: 34400351
- PMCID: PMC8714665
- DOI: 10.1016/j.drudis.2021.08.004
Vorinostat in autophagic cell death: A critical insight into autophagy-mediated, -associated and -dependent cell death for cancer prevention
Abstract
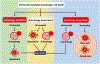
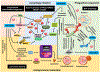
Histone deacetylases (HDACs) inhibit the acetylation of crucial autophagy genes, thereby deregulating autophagy and autophagic cell death (ACD) and facilitating cancer cell survival. Vorinostat, a broad-spectrum pan-HDAC inhibitor, inhibits the deacetylation of key autophagic markers and thus interferes with ACD. Vorinostat-regulated ACD can have an autophagy-mediated, -associated or -dependent mechanism depending on the involvement of apoptosis. Molecular insights revealed that hyperactivation of the PIK3C3/VPS34-BECN1 complex increases lysosomal disparity and enhances mitophagy. These changes are followed by reduced mitochondrial biogenesis and by secondary signals that enable superactivated, nonselective or bulk autophagy, leading to ACD. Although the evidence is limited, this review focuses on molecular insights into vorinostat-regulated ACD and describes critical concepts for clinical translation.
Keywords: Autophagy; Cancer; Cell death; Histone deacetylases; Vorinostat.
Copyright © 2021 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
Similar articles
-
Targeting SIRT1-regulated autophagic cell death as a novel therapeutic avenue for cancer prevention.Drug Discov Today. 2023 Sep;28(9):103692. doi: 10.1016/j.drudis.2023.103692. Epub 2023 Jun 26. Drug Discov Today. 2023. PMID: 37379905 Review.
-
Broad-Spectrum HDAC Inhibitors Promote Autophagy through FOXO Transcription Factors in Neuroblastoma.Cells. 2021 Apr 24;10(5):1001. doi: 10.3390/cells10051001. Cells. 2021. PMID: 33923163 Free PMC article.
-
Histone deacetylase inhibitors potentiate vesicular stomatitis virus oncolysis in prostate cancer cells by modulating NF-κB-dependent autophagy.J Virol. 2014 Mar;88(5):2927-40. doi: 10.1128/JVI.03406-13. Epub 2013 Dec 26. J Virol. 2014. PMID: 24371063 Free PMC article.
-
Curbing autophagy and histone deacetylases to kill cancer cells.Autophagy. 2012 Oct;8(10):1521-2. doi: 10.4161/auto.21151. Epub 2012 Aug 16. Autophagy. 2012. PMID: 22894919 Free PMC article.
-
p53 at the Crossroads between Different Types of HDAC Inhibitor-Mediated Cancer Cell Death.Int J Mol Sci. 2019 May 15;20(10):2415. doi: 10.3390/ijms20102415. Int J Mol Sci. 2019. PMID: 31096697 Free PMC article. Review.
Cited by
-
Pathogenesis of Hepatocellular Carcinoma: The Interplay of Apoptosis and Autophagy.Biomedicines. 2023 Apr 13;11(4):1166. doi: 10.3390/biomedicines11041166. Biomedicines. 2023. PMID: 37189787 Free PMC article. Review.
-
Epigenetic Regulation in Breast Cancer: Insights on Epidrugs.Epigenomes. 2023 Feb 18;7(1):6. doi: 10.3390/epigenomes7010006. Epigenomes. 2023. PMID: 36810560 Free PMC article. Review.
-
Carboranes as unique pharmacophores in antitumor medicinal chemistry.Mol Ther Oncolytics. 2022 Jan 10;24:400-416. doi: 10.1016/j.omto.2022.01.005. eCollection 2022 Mar 17. Mol Ther Oncolytics. 2022. PMID: 35141397 Free PMC article. Review.
-
Pan-histone deacetylase inhibitor vorinostat suppresses osteoclastic bone resorption through modulation of RANKL-evoked signaling and ameliorates ovariectomy-induced bone loss.Cell Commun Signal. 2024 Mar 4;22(1):160. doi: 10.1186/s12964-024-01525-w. Cell Commun Signal. 2024. PMID: 38439009 Free PMC article.
-
Natural Bioactive Compounds Targeting Histone Deacetylases in Human Cancers: Recent Updates.Molecules. 2022 Apr 15;27(8):2568. doi: 10.3390/molecules27082568. Molecules. 2022. PMID: 35458763 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

