Nitric oxide-releasing micelles with intelligent targeting for enhanced anti-tumor effect of cisplatin in hypoxia
- PMID: 34399762
- PMCID: PMC8365946
- DOI: 10.1186/s12951-021-00989-z
Nitric oxide-releasing micelles with intelligent targeting for enhanced anti-tumor effect of cisplatin in hypoxia
Abstract
Background: Hypoxic tumor microenvironment (TME) promotes tumor metastasis and drug resistance, leading to low efficiency of cancer chemotherapy. The development of targeted agents or multi-target therapies regulating hypoxic microenvironment is an important approach to overcome drug resistance and metastasis.
Methods: In this study, chitosan oligosaccharide (COS)-coated and sialic acid (SA) receptor-targeted nano-micelles were prepared using film dispersion method to co-deliver cisplatin (CDDP) and nitric oxide (NO) (denoted as CTP/CDDP). In addition, we explored the mechanisms by which NO reversed CDDP resistance as well as enhanced anti-metastatic efficacy in hypoxic cancer cells.
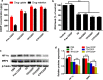
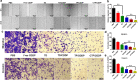
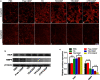
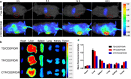
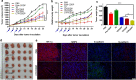
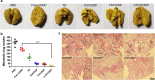
Results: Because of the different affinities of COS and SA to phenylboronic acid (PBA) under different pH regimes, CTP/CDDP micelles with intelligent targeting property increased cellular uptake of CDDP and enhanced cytotoxicity to tumors, but reduced systemic toxicity to normal organs or tissues. In addition, CTP/CDDP showed stimulus-responsive release in TME. In terms of anti-tumor mechanism, CTP/CDDP reduced CDDP efflux and inhibited epithelial-mesenchymal transition (EMT) process of tumor by down-regulating hypoxia-inducible factor-1α (HIF-1α), glutathione (GSH), multidrug resistance-associated protein 2 (MRP2) and matrix metalloproteinase 9 (MMP9) expression, thus reversing drug resistance and metastasis of hypoxic tumor cells.
Conclusions: The designed micelles significantly enhanced anti-tumor effects both in vitro and in vivo. These results suggested that CTP/CDDP represented a promising strategy to treat resistance and metastatic tumors.
Keywords: Chitosan oligosaccharide; Cisplatin; Drug resistance; HIF-1α; Hypoxia; Metastasis; Nitric oxide.
© 2021. The Author(s).
Conflict of interest statement
The authors have declared that no competing interest exists.
Figures
Similar articles
-
Up-regulation of survivin by AKT and hypoxia-inducible factor 1α contributes to cisplatin resistance in gastric cancer.FEBS J. 2014 Jan;281(1):115-28. doi: 10.1111/febs.12577. Epub 2013 Nov 18. FEBS J. 2014. PMID: 24165223
-
Hypoxia-induced increases in A549/CDDP cell drug resistance are reversed by RNA interference of HIF-1α expression.Mol Med Rep. 2012 Jan;5(1):228-32. doi: 10.3892/mmr.2011.604. Epub 2011 Sep 29. Mol Med Rep. 2012. PMID: 21964646
-
Overcoming chemotherapy resistance using pH-sensitive hollow MnO2 nanoshells that target the hypoxic tumor microenvironment of metastasized oral squamous cell carcinoma.J Nanobiotechnology. 2021 May 26;19(1):157. doi: 10.1186/s12951-021-00901-9. J Nanobiotechnology. 2021. PMID: 34039370 Free PMC article.
-
Tumor microenvironment and epithelial mesenchymal transition as targets to overcome tumor multidrug resistance.Drug Resist Updat. 2020 Dec;53:100715. doi: 10.1016/j.drup.2020.100715. Epub 2020 Jun 20. Drug Resist Updat. 2020. PMID: 32679188 Review.
-
Matrix metalloproteinases as the critical regulators of cisplatin response and tumor cell invasion.Eur J Pharmacol. 2024 Nov 5;982:176966. doi: 10.1016/j.ejphar.2024.176966. Epub 2024 Aug 30. Eur J Pharmacol. 2024. PMID: 39216742 Review.
Cited by
-
Drug-Loaded Chitosan Scaffolds for Periodontal Tissue Regeneration.Polymers (Basel). 2022 Aug 5;14(15):3192. doi: 10.3390/polym14153192. Polymers (Basel). 2022. PMID: 35956708 Free PMC article. Review.
-
Non-coding RNA and drug resistance in head and neck cancer.Cancer Drug Resist. 2024 Sep 20;7:34. doi: 10.20517/cdr.2024.59. eCollection 2024. Cancer Drug Resist. 2024. PMID: 39403599 Free PMC article. Review.
-
Delivery of Nitric Oxide in the Cardiovascular System: Implications for Clinical Diagnosis and Therapy.Int J Mol Sci. 2021 Nov 10;22(22):12166. doi: 10.3390/ijms222212166. Int J Mol Sci. 2021. PMID: 34830052 Free PMC article. Review.
-
Recent advances in anti-multidrug resistance for nano-drug delivery system.Drug Deliv. 2022 Dec;29(1):1684-1697. doi: 10.1080/10717544.2022.2079771. Drug Deliv. 2022. PMID: 35616278 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
- 2019CFB521/natural science foundation of hubei province
- 81973257/Programs of the National Natural Science Foundation of China
- 81673368/Programs of the National Natural Science Foundation of China
- 82073091/Programs of the National Natural Science Foundation of China
- 2020yjsCXCY047/Graduates' Innovation Fund of HUST
LinkOut - more resources
Full Text Sources
Miscellaneous

