Systemic corticosteroids for the treatment of COVID-19
- PMID: 34396514
- PMCID: PMC8406706
- DOI: 10.1002/14651858.CD014963
Systemic corticosteroids for the treatment of COVID-19
Update in
-
Systemic corticosteroids for the treatment of COVID-19: Equity-related analyses and update on evidence.Cochrane Database Syst Rev. 2022 Nov 17;11(11):CD014963. doi: 10.1002/14651858.CD014963.pub2. Cochrane Database Syst Rev. 2022. PMID: 36385229 Free PMC article. Review.
Abstract
Background: Systemic corticosteroids are used to treat people with COVID-19 because they counter hyper-inflammation. Existing evidence syntheses suggest a slight benefit on mortality. So far, systemic corticosteroids are one of the few treatment options for COVID-19. Nonetheless, size of effect, certainty of the evidence, optimal therapy regimen, and selection of patients who are likely to benefit most are factors that remain to be evaluated.
Objectives: To assess whether systemic corticosteroids are effective and safe in the treatment of people with COVID-19, and to keep up to date with the evolving evidence base using a living systematic review approach.
Search methods: We searched the Cochrane COVID-19 Study Register (which includes PubMed, Embase, CENTRAL, ClinicalTrials.gov, WHO ICTRP, and medRxiv), Web of Science (Science Citation Index, Emerging Citation Index), and the WHO COVID-19 Global literature on coronavirus disease to identify completed and ongoing studies to 16 April 2021.
Selection criteria: We included randomised controlled trials (RCTs) that evaluated systemic corticosteroids for people with COVID-19, irrespective of disease severity, participant age, gender or ethnicity. We included any type or dose of systemic corticosteroids. We included the following comparisons: systemic corticosteroids plus standard care versus standard care (plus/minus placebo), dose comparisons, timing comparisons (early versus late), different types of corticosteroids and systemic corticosteroids versus other active substances. We excluded studies that included populations with other coronavirus diseases (severe acute respiratory syndrome or Middle East respiratory syndrome), corticosteroids in combination with other active substances versus standard care, topical or inhaled corticosteroids, and corticosteroids for long-COVID treatment.
Data collection and analysis: We followed standard Cochrane methodology. To assess the risk of bias in included studies, we used the Cochrane 'Risk of bias' 2 tool for RCTs. We rated the certainty of evidence using the GRADE approach for the following outcomes: all-cause mortality, ventilator-free days, new need for invasive mechanical ventilation, quality of life, serious adverse events, adverse events, and hospital-acquired infections.
Main results: We included 11 RCTs in 8075 participants, of whom 7041 (87%) originated from high-income countries. A total of 3072 participants were randomised to corticosteroid arms and the majority received dexamethasone (n = 2322). We also identified 42 ongoing studies and 16 studies reported as being completed or terminated in a study registry, but without results yet. Hospitalised individuals with a confirmed or suspected diagnosis of symptomatic COVID-19 Systemic corticosteroids plus standard care versus standard care plus/minus placebo We included 10 RCTs (7989 participants), one of which did not report any of our pre-specified outcomes and thus our analysis included outcome data from nine studies. All-cause mortality (at longest follow-up available): systemic corticosteroids plus standard care probably reduce all-cause mortality slightly in people with COVID-19 compared to standard care alone (median 28 days: risk difference of 30 in 1000 participants fewer than the control group rate of 275 in 1000 participants; risk ratio (RR) 0.89, 95% confidence interval (CI) 0.80 to 1.00; 9 RCTs, 7930 participants; moderate-certainty evidence). Ventilator-free days: corticosteroids may increase ventilator-free days (MD 2.6 days more than control group rate of 4 days, 95% CI 0.67 to 4.53; 1 RCT, 299 participants; low-certainty evidence). Ventilator-free days have inherent limitations as a composite endpoint and should be interpreted with caution. New need for invasive ventilation: the evidence is of very low certainty. Because of high risk of bias arising from deaths that occurred before ventilation we are uncertain about the size and direction of the effects. Consequently, we did not perform analysis beyond the presentation of descriptive statistics. Quality of life/neurological outcome: no data were available. Serious adverse events: we included data on two RCTs (678 participants) that evaluated systemic corticosteroids compared to standard care (plus/minus placebo); for adverse events and hospital-acquired infections, we included data on five RCTs (660 participants). Because of high risk of bias, heterogeneous definitions, and underreporting we are uncertain about the size and direction of the effects. Consequently, we did not perform analysis beyond the presentation of descriptive statistics (very low-certainty evidence). Different types, dosages or timing of systemic corticosteroids We identified one study that compared methylprednisolone with dexamethasone. The evidence for mortality and new need for invasive mechanical ventilation is very low certainty due to the small number of participants (n = 86). No data were available for the other outcomes. We did not identify comparisons of different dosages or timing. Outpatients with asymptomatic or mild disease Currently, there are no studies published in populations with asymptomatic infection or mild disease.
Authors' conclusions: Moderate-certainty evidence shows that systemic corticosteroids probably slightly reduce all-cause mortality in people hospitalised because of symptomatic COVID-19. Low-certainty evidence suggests that there may also be a reduction in ventilator-free days. Since we are unable to adjust for the impact of early death on subsequent endpoints, the findings for ventilation outcomes and harms have limited applicability to inform treatment decisions. Currently, there is no evidence for asymptomatic or mild disease (non-hospitalised participants). There is an urgent need for good-quality evidence for specific subgroups of disease severity, for which we propose level of respiratory support at randomisation. This applies to the comparison or subgroups of different types and doses of corticosteroids, too. Outcomes apart from mortality should be measured and analysed appropriately taking into account confounding through death if applicable. We identified 42 ongoing and 16 completed but not published RCTs in trials registries suggesting possible changes of effect estimates and certainty of the evidence in the future. Most ongoing studies target people who need respiratory support at baseline. With the living approach of this review, we will continue to update our search and include eligible trials and published data.
Copyright © 2021 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
CW: is funded by the Federal Ministry of Education and Research, Germany (NaFoUniMedCovid19, funding number: 01KX2021; part of the project 'CEOSys', which was paid to the institution).
MG: is funded by the Federal Ministry of Education and Research, Germany (NaFoUniMedCovid19, funding number: 01KX2021; part of the project 'CEOSys', which was paid to the institution); works as a resident with the Department of Anaesthesiology and Intensive Care at the University of Leipzig Medical Center; is member of the German Society for Anaesthesia and Intensive Care.
AM: works as a physician at the Department of Infectious Diseases and Respiratory Medicine at Charité University medicine Berlin; is a member of the German Society for Infectious Diseases; works in the office of STAKOB at Robert Koch‐Institut; coordinates the work of the specialist group COVRIIN.
AMu: none known.
MM: is funded by the Federal Ministry of Education and Research, Germany (NaFoUniMedCovid19, funding number: 01KX2021; part of the project 'CEOSys', which was paid to the institution).
AF: is funded by the Federal Ministry of Education and Research, Germany (NaFoUniMedCovid19, funding number: 01KX2021; part of the project 'CEOSys', which was paid to the institution) and works as a resident with the Department of Anaesthesiology and Intensive Care at the University of Leipzig Medical Center.
MK: is funded by the Federal Ministry of Education and Research, Germany (NaFoUniMedCovid19, funding number: 01KX2021; part of the project 'CEOSys', which was paid to the institution).
MN: works as a health professional; Leadership or other fiduciary role in other board, society, committee, or advocacy group; Published opinions in medical journals, the public press, broadcast and social media relevant to the interventions in the work.
MS: none known.
KK: is funded by the Federal Ministry of Education and Research, Germany (NaFoUniMedCovid19, funding number: 01KX2021; part of the project 'CEOSys', which was paid to the institution); works as an intensive care specialist with the Department of Anaesthesiology and Intensive Care at the University of Leipzig Medical Center; is a member of the German Society for Anaesthesia and Intensive Care.
NS: none known.
FF: works as an intensive care consultant with the Department of Anaesthesiology and Intensive Care at the University of Leipzig Medical Center and is a member of the CEOsys project (no direct funding), the German Society for Anaesthesia and Intensive Care (DGAI), and the German Interdisciplinary Association for Intensive and Emergency Medicine (DIVI). Leading role in German guideline on respiratory failure and invasive mechanical ventilation.
Figures

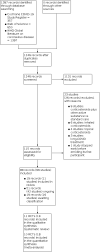













Similar articles
-
Systemic corticosteroids for the treatment of COVID-19: Equity-related analyses and update on evidence.Cochrane Database Syst Rev. 2022 Nov 17;11(11):CD014963. doi: 10.1002/14651858.CD014963.pub2. Cochrane Database Syst Rev. 2022. PMID: 36385229 Free PMC article. Review.
-
Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas.Cochrane Database Syst Rev. 2022 Feb 1;2(2022):CD014217. doi: 10.1002/14651858.CD014217. Cochrane Database Syst Rev. 2022. PMID: 36321557 Free PMC article.
-
Colchicine for the treatment of COVID-19.Cochrane Database Syst Rev. 2021 Oct 18;10(10):CD015045. doi: 10.1002/14651858.CD015045. Cochrane Database Syst Rev. 2021. PMID: 34658014 Free PMC article. Review.
-
Inhaled corticosteroids for the treatment of COVID-19.Cochrane Database Syst Rev. 2022 Mar 9;3(3):CD015125. doi: 10.1002/14651858.CD015125. Cochrane Database Syst Rev. 2022. PMID: 35262185 Free PMC article. Review.
-
Remdesivir for the treatment of COVID-19.Cochrane Database Syst Rev. 2023 Jan 25;1(1):CD014962. doi: 10.1002/14651858.CD014962.pub2. Cochrane Database Syst Rev. 2023. PMID: 36695483 Free PMC article. Review.
Cited by
-
Non-Dexamethasone Corticosteroid Therapy's Effect on COVID-19 Prognosis in Cancer Patients: A Retrospective Study.Vaccines (Basel). 2023 Jan 28;11(2):290. doi: 10.3390/vaccines11020290. Vaccines (Basel). 2023. PMID: 36851168 Free PMC article.
-
Real-world Evidence of the Effects of Novel Treatments for COVID-19 on Mortality: A Nationwide Comparative Cohort Study of Hospitalized Patients in the First, Second, Third, and Fourth Waves in the Netherlands.Open Forum Infect Dis. 2022 Nov 22;9(12):ofac632. doi: 10.1093/ofid/ofac632. eCollection 2022 Dec. Open Forum Infect Dis. 2022. PMID: 36519114 Free PMC article.
-
Steroid Pulse Therapy as a Treatment for Patients With COVID-19 Pneumonia at an Intensive Care Unit: A Single-Center Retrospective Observational Study.Cureus. 2023 Mar 20;15(3):e36386. doi: 10.7759/cureus.36386. eCollection 2023 Mar. Cureus. 2023. PMID: 36945235 Free PMC article.
-
Molecular Insights of SARS-CoV-2 Antivirals Administration: A Balance between Safety Profiles and Impact on Cardiovascular Phenotypes.Biomedicines. 2022 Feb 14;10(2):437. doi: 10.3390/biomedicines10020437. Biomedicines. 2022. PMID: 35203646 Free PMC article. Review.
-
Prolonged SARS-CoV-2 infection during autologous hematopoietic stem cell transplantation from Hodgkin's lymphoma: a case report.Pan Afr Med J. 2023 Mar 16;44:133. doi: 10.11604/pamj.2023.44.133.36277. eCollection 2023. Pan Afr Med J. 2023. PMID: 37333782 Free PMC article.
References
References to studies included in this review
Angus 2020 {published data only}
-
- Angus DC, Berry S, Lewis RJ, Al-Beidh F, Arabi Y, Van Bentum-Puijk W, et al. The randomised embedded multifactorial adaptive platform for community-acquired pneumonia (REMAP-CAP) study: rationale and design. Annals of the American Thoracic Society 2020;17(7):879-91. [DOI: 10.1513/AnnalsATS.202003-192SD] - DOI - PMC - PubMed
-
- Angus DC, Derde L, Al-Beidh F, Annane D, Arabi Y, Beane A, et al. Effect of hydrocortisone on mortality and organ support in patients with severe COVID-19: the REMAP-CAP COVID-19 corticosteroid domain randomised clinical trial. JAMA 2020;324(13):1317-29. [DOI: 10.1001/jama.2020.17022] [NCT02735707] - DOI - PMC - PubMed
Corral‐Gudino 2021 {published data only}
-
- Corral-Gudino L, Bahamonde A, Arnaiz-Revillas F, Gómez-Barquero J, Abadía-Otero J, García-Ibarbia C, et al. Methylprednisolone in adults hospitalised with COVID-19 pneumonia: an open-label randomised trial (GLUCOCOVID). Wiener Klinische Wochenschrift 2021;133(7-8):303-11. [DOI: 10.1007/s00508-020-01805-8] [EUCTR2020-001934-37-ES] - DOI - PMC - PubMed
-
- Corral L, Bahamonde A, Arnaiz delas Revillas F, Gomez-Barquero J, Abadia-Otero J, Garcia-Ibarbia C, et al. GLUCOCOVID: a controlled trial of methylprednisolone in adults hospitalised with COVID-19 pneumonia. medRxiv [Preprint]. [DOI: 10.1101/2020.06.17.20133579] [EUCTR2020-001934-37-ES] - DOI - PubMed
Dequin 2020 {published data only}
-
- Dequin PF, Gouge AL, Tavernier E, Giraudeau B, Zohar S. Embedding a COVID-19 group sequential clinical trial within an ongoing trial: lessons from an unusual experience. Statistics in Biopharmaceutical Research 2020:1-5. [EMBASE: https://www.embase.com/search/results?subaction=viewrecord&id=L200590637...] - PMC - PubMed
-
- Dequin PF, Heming N, Meziani F, Plantefève G, Voiriot G, Badié J, et al. Effect of hydrocortisone on 21-day mortality or respiratory support among critically ill patients with COVID-19: a randomised clinical trial. JAMA 2020;324(13):1298-306. [DOI: 10.1001/jama.2020.16761] [NCT02517489] - DOI - PMC - PubMed
Edalatifard 2020 {published data only}
-
- Edalatifard M, Akhtari M, Salehi M, Naderi Z, Jamshidi A, Mostafaei S, et al. Intravenous methylprednisolone pulse as a treatment for hospitalised severe COVID-19 patients: results from a randomised controlled clinical trial. European Respiratory Journal 2020;56(6):1-13. [DOI: 10.1183/13993003.02808-2020] [IRCT20200404046947N1] - DOI - PMC - PubMed
Farahani 2021 {published data only}
-
- Farahani RH, Mosaed R, Nezami-Asl A, Chamanara M, Soleiman-Meigooni S, Kalantar S, et al. Evaluation of the efficacy of methylprednisolone pulse therapy in treatment of COVID-19 adult patients with severe respiratory failure: randomised, clinical trial. Research Square 2021;Version 1:1-19. [DOI: 10.21203/rs.3.rs-66909/v1] [IRCT20200406046963N1] - DOI
Horby 2021 {published data only}
-
- Horby P, Lim WS, Emberson J, Mafham M, Bell J, Linsell L, et al. Effect of dexamethasone in hospitalised patients with COVID-19: preliminary report. medRxiv [Preprint] 2020. [DOI: ]
-
- Oxford University. Low-cost dexamethasone reduces death by up to one third in hospitalised patients with severe respiratory complications of COVID-19. University of Oxford 2020.
Jamaati 2021 {published data only}
-
- Jamaati H, Hashemian SM, Farzanegan B, Malekmohammad M, Tabarsi P, Marjani M, et al. No clinical benefit of high dose corticosteroid administration in patients with COVID-19: a preliminary report of a randomised clinical trial. European Journal of Pharmacology 2021;897:173947. [DOI: 10.1016/j.ejphar.2021.173947] [IRCT20151227025726N17] - DOI - PMC - PubMed
Jeronimo 2020 {published data only}
-
- Jeronimo CM, Farias ME, Val FF, Sampaio VS, Alexandre MA, Melo GC, et al. Methylprednisolone as adjunctive therapy for patients hospitalised with COVID-19 (metcovid): a randomised, double-blind, phase IIb, placebo-controlled trial. Clinical Infectious Diseases 2020;72(9):e373–e81. [DOI: 10.1093/cid/ciaa1177] [NCT04343729] - DOI - PMC - PubMed
Ranjbar 2021 {published data only}
-
- Ranjbar K, Shahriarirad R, Erfani A, Khodamoradi Z, Saadi MHG, et al. Methylprednisolone or dexamethasone, which one is the superior corticosteroid in the treatment of hospitalised COVID-19 Patients: a triple-blinded randomised controlled trial. Research Square (Preprint). [DOI: ] [IRCT20200204046369N1] - PubMed
Tang 2021 {published data only}
-
- Tang X, Feng YM, Ni JX, Zhang JY, Liu LM, Hu K, et al. Early use of corticosteroid may prolong SARS-CoV-2 shedding in non-intensive care unit patients with COVID-19 pneumonia: a multicenter, single-blind, randomised control trial. Respiration 2021;100(2):116-26. [DOI: 10.1159/000512063] [NCT04273321] - DOI - PMC - PubMed
Tomazini 2020 {published data only}
-
- Tomazini BM, Maia IS, Bueno FR, Silva Mvao Baldassare FP, Costa EL, Moura RA, et al. COVID-19-associated ARDS treated with dexamethasone (CoDEX): study design and rationale for a randomised trial. Revista Brasileira de Terapia Intensiva 2020;32(3):354-62. [DOI: 10.5935/0103-507X.20200063] - DOI - PMC - PubMed
-
- Tomazini BM, Maia IS, Cavalcanti AB, Berwanger O, Rosa RG, Veiga VC, et al. Effect of dexamethasone on days alive and ventilator-free in patients with moderate or severe acute respiratory distress syndrome and COVID-19: the CoDEX randomised clinical trial. JAMA 2020;324(13):1307-16. [DOI: 10.1001/jama.2020.17021] [NCT04327401] - DOI - PMC - PubMed
References to studies excluded from this review
EUCTR2020‐001445‐39‐ES {published data only}
-
- EUCTR2020-001445-39-ES. Clinical trial to evaluate methylprednisolone pulses and tacrolimus in hospitalized patients with severe pneumonia secondary to COVID-19 (tacrovid). www.clinicaltrialsregister.eu/ctr-search/search?query=eudract_number:202... (first received 31 March 2020).
EUCTR2020‐001616‐18‐ES {published data only}
-
- Treatment with inhaled corticoids in patients with COVID-19 and pneumonia. www.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2020-001616-18-ES.
EUCTR2020‐001889‐10 {published data only}
-
- EUCTR2020-001889-10. Use of inhaled corticosteroids as treatment of early COVID-19 infection to prevent clinical deterioration and hospitalisation. www.clinicaltrialsregister.eu/ctr-search/trial/2020-001889-10/GB (first received 15 May 2020).
IRCT20120225009124N4 {published data only}
-
- IRCT20120225009124N4. Letter to the editor: efficacy of different methods of combination regimen administrations including dexamethasone, intravenous immunoglobulin, and interferon-beta to treat critically ill COVID-19 patients: a structured summary of a study protocol for a randomized controlled trial. Trials 2020;21(1):549. [DOI: 10.1186/s13063-020-04499-5] - DOI - PMC - PubMed
IRCT20190312043030N2 {published data only}
-
- IRCT20190312043030N2. The effect of selenium, vitamin C and methylprednisolone combination on mortality and morbidity of COVID-19 patients. en.irct.ir/trial/49508 (first received 19 August 2020).
IRCT20200522047542N1 {published data only}
-
- IRCT20200522047542N1. Effect of corton on olfactory dysfunction in COVID-19 patients. en.irct.ir/trial/48379 (first received 4 August 2020).
ISRCTN86534580 {published data only}
-
- ISRCTN86534580. PRINCIPLE: a trial evaluating treatments for suspected COVID-19 in people aged 50 years and above with pre-existing conditions and those aged 65 years and above. www.isrctn.com/ISRCTN86534580 (first received 20 March 2020).
NCT04341038 {published data only}
-
- NCT04341038. Clinical trial to evaluate methylprednisolone pulses and tacrolimus in patients with COVID-19 lung injury. clinicaltrials.gov/show/NCT04341038 (first received 10 April 2020).
-
- NCT04341038. Pragmatic, open-label, single-center, randomized, phase II clinical trial to evaluate the efficacy and safety of methylprednisolone pulses and tacrolimus in patients with severe pneumonia secondary to COVID-19: the TACROVID trial protocol. medRxiv [Preprint] 2021;21(100716):1-8. [DOI: 10.1016/j.conctc.2021.100716] - DOI - PMC - PubMed
NCT04355637 {published data only}
-
- NCT04355637. Inhaled corticosteroid treatment of COVID-19 patients with pneumonia. clinicaltrials.gov/show/NCT04355637 (first received 21 April 2020).
NCT04359511 {published data only}
-
- NCT04359511. Efficacy and safety of corticosteroids in oxygen-dependent patients with COVID-19 pneumonia. clinicaltrials.gov/show/NCT04359511 (first received 24 April 2020).
NCT04361474 {published data only}
-
- Daval M, Corre A, Palpacuer C, Houssette J, Poillon G, Eliezer M, et al. Efficacy of local budesonide therapy in the management of persistent hyposmia in COVID-19 patients without signs of severity: a structured summary of a study protocol for a randomised controlled trial. Trials 2020;21(666):1-3. [DOI: ] - PMC - PubMed
-
- NCT04361474. Trial evaluating the efficacy of local budesonide therapy in the management of hyposmia in COVID-19 patients without signs of severity. clinicaltrials.gov/show/NCT04361474 (first received 24 April 2020).
NCT04381364 {published data only}
-
- NCT04381364. Inhalation of ciclesonide for patients with COVID-19: a randomised open treatment study (HALT COVID-19). clinicaltrials.gov/show/NCT04381364 (first received 8 May 2020).
NCT04411667 {published data only}
NCT04416399 {published data only}
NCT04468646 {published data only}
-
- Riffat M, Fridoon A, Ahad Q, Muhammad AR, Syed AG, Muhammad AT, et al. Aprepitant as a combinant with dexamethasone reduces the inflammation via neurokinin 1 receptor antagonism in severe to critical COVID-19 patients and potentiates respiratory recovery: a novel therapeutic approach. medRxiv [Preprint]. [DOI: ]
NCT04484493 {published data only}
NCT04534478 {published data only}
-
- NCT04534478. Oral prednisone regimens to optimise the therapeutic strategy in patients with organising pneumonia post-COVID-19. clinicaltrials.gov/show/NCT04534478 (first received 1 September 2020).
NCT04551781 {unpublished data only}
-
- NCT04551781. Short term low dose corticosteroids for management of post COVID19 pulmonary fibrosis. clinicaltrials.gov/show/NCT04551781 (first received 16 September 2020).
NCT04561180 {published data only}
-
- NCT04561180. Study to evaluate the efficacy and safety of EG-HPCP-03a compared to DEX in patients with COVID-19 pneumonia. clinicaltrials.gov/show/NCT04561180 (first received 23 September 2020).
NCT04569825 {published data only}
-
- NCT04569825. Effect of nasal steroid in the treatment of anosmia due to COVID-19 disease. clinicaltrials.gov/ct2/show/NCT04569825 (first received 30 September 2020).
NCT04640168 {published data only}
-
- NCT04640168. Adaptive COVID-19 treatment trial 4 (ACTT-4). clinicaltrials.gov/show/NCT04640168 (first received 23 November 2020).
NCT04657484 {published data only}
-
- NCT04657484. Comparison of two corticosteroid regimens for post-COVID diffuse lung disease. clinicaltrials.gov/show/NCT04657484 (first received 8 December 2020).
NCT04826822 {published data only}
-
- NCT04826822. Spironolactone and dexamethasone in patients hospitalised with COVID-19 (SPIDEX-II). clinicaltrials.gov/show/NCT04826822 (first received 1 April 2021).
References to studies awaiting assessment
EUCTR2020‐001307‐16‐ES {published data only}
-
- EUCTR2020-001307-16-ES. Efficacy and safety of corticoids in patients with adult respiratory distress syndrome (ARDS) secondary to coronavirus infection. www.clinicaltrialsregister.eu/ctr-search/search?query=eudract_number:202... (first received 1 April 2020).
EUCTR2020‐001333‐13‐FR {published data only}
-
- EUCTR2020-001333-13-FR. Dexamethasone associated with hydroxychloroquine vs. hydroxychloroquine alone for the early treatment of severe ARDS caused by COVID-19: a randomised controlled trial. www.clinicaltrialsregister.eu/ctr-search/search?query=eudract_number:202... (first received 9 April 2020).
EUCTR2020‐001553‐48‐FR {published data only}
-
- EUCTR2020-001553-48-FR. Corticoids in COVID-19 viral pneumonia in infection with SARS-CoV-2 (translation by the review authors) [Corticoides au cours de la pneumonie virale COVID-19 liee a linfection par le SARS-CoV-2]. www.clinicaltrialsregister.eu/ctr-search/search?query=eudract_number:202... (first received 13 April 2020).
IRCT20081027001411N3 {published data only}
-
- IRCT20081027001411N3. Effect of prednisolone on treatment of COVID-19. irct.ir/trial/46975 (first received 6 January 2020).
IRCT20100228003449N31 {published data only}
-
- IRCT20100228003449N31. Corticosteroids in COVID-19. irct.ir/trial/51163 (first received 8 October 2020).
IRCT20120215009014N354 {published data only}
-
- IRCT20120215009014N354. Evaluating the effect of intravenous hydrocortisone, methylprednisolone, and dexamethasone in treatment of patients with moderate to severe acute respiratory distress syndrome caused by COVID-19. irct.ir/trial/48043 (first received 12 May 2020).
IRCT20160118026097N4 {published data only}
-
- IRCT20160118026097N4. The effect of dexamethasone in the treatment of high-risk COVID-19 patients. irct.ir/trial/48310 (first received 13 September 2020).
IRCT20200611047727N3 {published data only}
-
- IRCT20200611047727N3. Evaluation of efficacy and safety of low dose corticosteroid with severe pneumonia COVID-19. irct.ir/trial/49116 (first received 3 January 2021).
IRCT20201015049030N1 {published data only}
-
- IRCT20201015049030N1. Effect of dexamethasone on treatment of COVID-19. www.irct.ir/trial/51736 (first received 7 November 2020).
ISRCTN33037282 {published data only}
-
- ISRCTN33037282. Comparing two medications (dexamethasone and methylprednisolone high dose) for the treatment of pneumonia in patients with COVID-19. www.isrctn.com/ISRCTN33037282 (first received 26 November 2020).
Munch 2021 {published data only}
-
- Munch MW, Meyhoff TS, Helleberg M, Kjær M-BN, Granholm A, Hjortsø CJ, et al. Low-dose hydrocortisone in patients with COVID-19 and severe hypoxia: the COVID STEROID randomised, placebo-controlled trial. Acta Anaesthesiologica Scandinavica 2021 Jun 17 [Epub ahead of print]. [DOI: 10.1111/aas.13941] - DOI - PMC - PubMed
-
- NCT04348305. Hydrocortisone for COVID-19 and severe hypoxia. clinicaltrials.gov/show/NCT04348305 (first received 16 April 2020).
-
- Petersen MW, Meyhoff TS, Helleberg M, Nørregaard Kjær MB, Granholm A, Steensen Hjortsø CJ, et al. Low-dose hydrocortisone in patients with COVID-19 and severe hypoxia (COVID STEROID) trial-protocol and statistical analysis plan. Acta Anaesthesiologica Scandinavica 2020;64(9):1365-75. [DOI: 10.1111/aas.13673] - DOI - PMC - PubMed
NCT03852537 {published data only}
-
- NCT03852537. Steroid dosing by biomarker guided titration in critically ill patients with pneumonia. clinicaltrials.gov/show/NCT03852537 (first received 25 February 2019).
NCT04244591 {published data only}
-
- NCT04244591. Glucocorticoid therapy for novel coronavirus critically ill patients with severe acute respiratory failure. www.clinicaltrials.gov/ct2/show/NCT04244591 (first received 28 January 2020).
NCT04325061 {published data only}
-
- NCT04325061. Efficacy of dexamethasone treatment for patients with ARDS caused by COVID-19. clinicaltrials.gov/ct2/show/NCT04325061 (first received 27 March 2020).
NCT04746430 {published data only}
-
- NCT04746430. COVID-19 primary care platform for early treatment and recovery (COPPER) study. clinicaltrials.gov/show/NCT04746430 (first received 9 February 2021).
Rashad 2021 {unpublished data only}
-
- NCT04519385. Tocilizumab versus dexamethasone in severe COVID19 cases. clinicaltrials.gov/show/NCT04519385 (first received 19 August 2020).
References to ongoing studies
ChiCTR2000029386 {published data only}
-
- Qin YY, Zhou YH, Lu YQ, Sun F, Yang S, Harypursat V, et al. Effectiveness of glucocorticoid therapy in patients with severe novel coronavirus pneumonia: protocol of a randomised controlled trial. Chinese Medical Journal 2020;133(9):1080-6. [DOI: 10.1097/CM9.0000000000000791] [ChiCTR2000029386] - DOI - PMC - PubMed
ChiCTR2000029656 {published data only}
-
- ChiCTR2000029656. A randomised, open-label study to evaluate the efficacy and safety of low-dose corticosteroids in hospitalised patients with novel coronavirus pneumonia (COVID-19). www.chictr.org.cn/showprojen.aspx?proj=49086 (first received 9 February 2020).
ChiCTR2000030481 {published data only}
-
- ChiCTR2000030481. The clinical value of corticosteroid therapy timing in the treatment of novel coronavirus pneumonia (COVID-19): a prospective randomised controlled trial. www.chictr.org.cn/showprojen.aspx?proj=50453 (first received 3 March 2020).
CTRI/2020/07/026608 {published data only}
-
- CTRI/2020/07/026608. A clinical trial to study the effects of two drugs methylprednisolone and dexamethasone in patients with severe COVID-19. www.ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=45638 (first received 15 July 2020).
CTRI/2020/10/028731 {published data only}
-
- CTRI/2020/10/028731. Higher vs. lower doses of dexamethasone in patients with COVID-19 and severe hypoxia. ctri.nic.in/Clinicaltrials/showallp.php?mid1=46442&EncHid=&userName=28731 (first received 29 October 2020).
-
- CTRI/2020/10/028731. Higher vs. lower doses of steroids in patients with COVID-19. www.who.int/trialsearch/Trial2.aspx?TrialID=CTRI/2020/10/028731 (first received 29 October 2020).
CTRI/2020/12/029894 {published data only}
-
- Comparing the effectiveness of dexamethasone versus methylprednisolone in patients with moderate COVID 19 - a randomised controlled trial. ctri.nic.in/Clinicaltrials/showallp.php?mid1=49273&EncHid=&userName=029894 (first received 18 December 2020).
-
- CTRI/2020/12/029894. A study to compare the effectiveness of two drugs, dexamethasone versus methylprednisolone in the treatment of moderate Covid 19 patients. www.who.int/trialsearch/Trial2.aspx?TrialID=CTRI/2020/12/029894.
CTRI/2020/12/030143 {published data only}
-
- CTRI/2020/12/030143. Comparison of different steroid regimes in critically ill adult patients of COVID-19. www.who.int/trialsearch/Trial2.aspx?TrialID=CTRI/2020/12/030143.
-
- Evaluation of different steroid regimes in critically ill adult patients of COVID-19 admitted to intensive care units. ctri.nic.in/Clinicaltrials/showallp.php?mid1=50886&EncHid=&userName=30143 (first received 31 December 2020).
EUCTR2020‐001413‐20‐ES {published data only}
-
- EUCTR2020-001413-20-ES. Efficacy and safety of siltuximab vs. corticosteroids in hospitalized patients with COVID-19 pneumonia. www.clinicaltrialsregister.eu/ctr-search/search?query=eudract_number:202... (first received 7 April 2020).
EUCTR2020‐001457‐43‐FR {published data only}
-
- EUCTR2020-001457-43-FR. Dexamethasone and oxygen support strategies in ICU patients with COVID-19 pneumonia. www.clinicaltrialsregister.eu/ctr-search/search?query=eudract_number:202... (first received 10 April 2020).
EUCTR2020‐001622‐64‐ES {published data only}
-
- EUCTR2020-001622-64-ES. Outpatient treatment of COVID-19 with early pulmonary corticosteroids as an opportunity to modify the course of the disease. www.clinicaltrialsregister.eu/ctr-search/search?query=eudract_number:202... (first received 19 April 2020).
-
- Saiz-Rodríguez M, Peña T, Lázaro L, González A, Martínez A, Cordero JA, et al. Outpatient treatment of COVID-19 with steroids in the phase of mild pneumonia without the need for admission as an opportunity to modify the course of the disease: a structured summary of a randomised controlled trial. Trials 2020;21(632):1-3. [DOI: 10.1186/s13063-020-04575-w] - DOI - PMC - PubMed
EUCTR2020‐001707‐16‐ES {published data only}
-
- EUCTR2020-001707-16-ES. Phase III randomised, unicentric open, controlled clinical trial to demonstrate the effectiveness of tocilizumab against systemic corticotherapy in patients entered by COVID-19 with bilateral pneumonia and bad evolution. www.clinicaltrialsregister.eu/ctr-search/trial/2020-001707-16/ES (first received 25 June 2020).
EUCTR2020‐001921‐30 {published data only}
-
- Busani S, Tosi M, Mighali P, Vandelli P, D'Amico R, Marietta M, et al. Multi-centre, three arm, randomised controlled trial on the use of methylprednisolone and unfractionated heparin in critically ill ventilated patients with pneumonia from SARS-CoV-2 infection: a structured summary of a study protocol for a randomised controlled trial. Trials 2020;21(1):724. [DOI: 10.1186/s13063-020-04645-z] - DOI - PMC - PubMed
-
- EUCTR2020-001921-30. Steroids and unfractionated heparin in critically-ill patients with pneumonia from COVID-19 infection. A multicenter, interventional, randomised, three arms study design. www.clinicaltrialsregister.eu/ctr-search/search?query=eudract_number:202... (first received 26 June 2020).
EUCTR2020‐002186‐34‐ES {published data only}
-
- EUCTR2020-002186-34-ES. Efficacy of the early use of corticotherapy in CoV-2 infection to prevent the progression of acute respiratory distress syndrome (ARDS). www.clinicaltrialsregister.eu/ctr-search/search?query=eudract_number:202... (first received 22 July 2020).
EUCTR2020‐003363‐25‐DK {published data only}
-
- EUCTR2020-003363-25-DK. Higher vs. lower doses of dexamethasone in patients with COVID-19 and severe oxygen deficiency: the COVID STEROID 2 trial. www.clinicaltrialsregister.eu/ctr-search/search?query=eudract_number:202... (first received 18 August 2020).
EUCTR2020‐004323‐16 {published data only}
-
- EUCTR2020-004323-16. Evaluation of the efficacy of high doses of methylprednisolone in SARS-CoV2 pneumonia patients. www.clinicaltrialsregister.eu/ctr-search/trial/2020-004323-16/IT (first received 23 November 2020).
NCT04329650 {published data only}
-
- NCT04329650. Efficacy and safety of siltuximab vs. corticosteroids in hospitalised patients with COVID19 pneumonia. clinicaltrials.gov/show/NCT04329650 (first received 1 April 2020).
NCT04344730 {published data only}
-
- NCT04344730. Dexamethasone and oxygen support strategies in ICU patients with COVID-19 pneumonia. clinicaltrials.gov/show/NCT04344730 (first received 14 April 2020).
NCT04345445 {published data only}
-
- NCT04345445. Study to evaluate the efficacy and safety of tocilizumab versus corticosteroids in hospitalised COVID-19 patients with high risk of progression. clinicaltrials.gov/show/NCT04345445 (first received 14 April 2020).
NCT04347980 {published data only}
-
- NCT04347980. Dexamethasone treatment for severe acute respiratory distress syndrome. clinicaltrials.gov/show/NCT04347980 (first received 15 April 2020).
NCT04377503 {published data only}
-
- NCT04377503. Tocilizumab versus methylprednisolone in the cytokine release syndrome of patients with COVID-19. clinicaltrials.gov/show/NCT04377503 (first received 6 May 2020).
NCT04395105 {published data only}
-
- Maskin LP, Olarte GL, Palizas F Jr, Velo AE, Lurbet MF, Bonelli I, et al. High dose dexamethasone treatment for acute respiratory distress syndrome secondary to COVID-19: a structured summary of a study protocol for a randomised controlled trial. Trials 2020;21(1):743. [DOI: 10.1186/s13063-020-04646-y] - DOI - PMC - PubMed
-
- NCT04395105. Dexamethasone for COVID-19 related ARDS: a multicenter, randomised clinical trial. clinicaltrials.gov/show/NCT04395105 (first received 20 May 2020).
NCT04438980 {published data only}
-
- Les Bujanda I, Loureiro-Amigo J, Capdevila Bastons F, Elejalde Guerra I, Anniccherico Sánchez J, Murgadella-Sancho A, et al. Treatment of COVID-19 pneumonia with glucocorticoids (CORTIVID): a structured summary of a study protocol for a randomised controlled trial. Trials 2021;22(1):43. [DOI: 10.1186/s13063-020-04999-4] - DOI - PMC - PubMed
-
- NCT04438980. Glucocorticoids in COVID-19 (CORTIVID). clinicaltrials.gov/show/NCT04438980 (first received 19 June 2020).
NCT04451174 {published data only}
-
- NCT04451174. Early use of corticosteroids in hospitalised patients with moderate COVID19 pneumonia. clinicaltrials.gov/show/NCT04451174 (first received 30 June 2020).
-
- Salinas M, Andino P, Palma L, Valencia J, Figueroa E, Ortega J. Early use of corticosteroids in non-critical patients with COVID-19 pneumonia (PREDCOVID): a structured summary of a study protocol for a randomised controlled trial. Trials 2021;22(1):92. [DOI: 10.1186/s13063-021-05046-6.] - DOI - PMC - PubMed
NCT04452565 {published data only}
-
- NCT04452565. NA-831, atazanavir and dexamethasone in the treatment of SARSCov-2 infection (NATADEX). clinicaltrials.gov/show/NCT04452565 (first received 30 June 2020).
NCT04485429 {published data only}
-
- NCT04485429. Efficacy assessment of methylprednisolone and heparin in patients with COVID-19 pneumonia. clinicaltrials.gov/show/NCT04485429 (first received 24 July 2020).
NCT04499313 {published data only}
-
- NCT04499313. Dexamethasone versus methylprednisolone for the treatment of patients with ARDS caused by COVID-19. clinicaltrials.gov/show/NCT04499313 (first received 5 August 2020).
NCT04509973 {published data only}
-
- Munch MW, Granholm A, Myatra SN, Vijayaraghavan BK, Cronhjort M, Wahlin RR, et al. Higher vs. lower doses of dexamethasone in patients with COVID-19 and severe hypoxia (COVID STEROID 2) trial: protocol and statistical analysis plan. Acta Anaesthesiologica Scandinavica 2021;00:1-12. [DOI: 10.1111/aas.13795] - DOI - PMC - PubMed
-
- NCT04509973. Higher vs. lower doses of dexamethasone for COVID-19 and severe hypoxia. clinicaltrials.gov/show/NCT04509973 (first received 12 August 2020).
NCT04513184 {published data only}
-
- NCT04513184. Randomised clinical trial of nasal dexamethasone as an adjuvant in patients with COVID-19. clinicaltrials.gov/show/NCT04513184 (first received 14 August 2020).
NCT04528329 {published data only}
-
- NCT04528329. Anosmia and / or ageusia and early corticosteroid use. clinicaltrials.gov/show/NCT04528329 (first received 27 August 2020).
NCT04528888 {published data only}
-
- NCT04528888. Steroids and unfractionated heparin in critically ill patients with pneumonia from COVID-19 infection. clinicaltrials.gov/show/NCT04528888 (first received 27 August 2020). [EUDRA-CT: https://www.clinicaltrialsregister.eu/ctr-search/trial/2020-001921-30/IT] [NCT: https://clinicaltrials.gov/ct2/show/study/NCT04528888]
NCT04530409 {published data only}
-
- NCT04530409. Timing of corticosteroids in COVID-19. clinicaltrials.gov/show/NCT04530409 (first received 28 August 2020).
NCT04545242 {published data only}
-
- NCT04545242. Efficacy of dexamethasone in patients with acute hypoxemic respiratory failure caused by infections. clinicaltrials.gov/show/NCT04545242 (first received 10 September 2020).
NCT04636671 {published data only}
-
- NCT04636671. Methylprednisolone vs. dexamethasone in COVID-19 pneumonia (MEDEAS RCT). clinicaltrials.gov/show/NCT04636671 (first received 19 November 2020).
NCT04663555 {published data only}
-
- NCT04663555. Effect of two different doses of dexamethasone in patients with ARDS and COVID-19. clinicaltrials.gov/show/NCT04663555 (first received 11 December 2020).
NCT04673162 {published data only}
-
- NCT04673162. Evaluation of the efficacy of high doses of methylprednisolone in SARS-CoV2 ( COVID-19) pneumonia patients. clinicaltrials.gov/show/NCT04673162 (first received 17 December 2020).
NCT04707534 {published data only}
-
- NCT04707534. Dexamethasone for COVID-19. clinicaltrials.gov/show/NCT04707534 (first received 13 January 2021).
NCT04726098 {published data only}
-
- NCT04726098. Low or high dose of dexamethasone in patients with respiratory failure by COVID-19. clinicaltrials.gov/ct2/show/record/NCT04726098 (first received 27 January 2021).
NCT04765371 {published data only}
-
- NCT04765371. Comparison between prednisolone and dexamethasone on mortality in patients on oxygen therapy, with COVID-19. clinicaltrials.gov/ct2/show/NCT04765371 (first received 21 February 2021).
NCT04780581 {published data only}
-
- NCT04780581. Glucocorticoid therapy in coronavirus disease COVID-19 patients. clinicaltrials.gov/show/NCT04780581 (first received 3 March 2021).
NCT04795583 {published data only}
-
- NCT04795583. Corticosteroids for COVID-19. clinicaltrials.gov/show/NCT04795583 (first received 12 March 2021).
NCT04834375 {published data only}
-
- NCT04834375. Randomised open investigation determining steroid dose. clinicaltrials.gov/show/NCT04834375 (first received 8 April 2021).
NCT04836780 {published data only}
-
- NCT04836780. Dexamethasone early administration in hospitalised patients with COVID-19 pneumonia. clinicaltrials.gov/show/NCT04836780 (first received 8 April 2021).
Additional references
Barnes 2006
Bourdeau 2003
-
- Bourdeau I, Stratakis CA. Glucocorticoids, Pharmacology of. In: Henry H, Norman A, editors(s). Encyclopedia of Hormones. Vol. -. Cambridge, MA: Academic Press, 2003:142-50. [DOI: ]
Brock 2011
Buitrago‐Garcia 2020
Chaudhuri 2021
Columbia Public Health 2021
-
- Competing Risk Analysis. Columbia University Mailman School of Public Health Website. Available from www.publichealth.columbia.edu/research/population-health-methods/competi... (accessed 13 July 2021). [ACCESSED JULY 13 2021: https://www.publichealth.columbia.edu/research/population-health-methods...]
COMET 2020
-
- Core outcome set developers’ response to COVID-19. www.comet-initiative.org/Studies/Details/1538 (accessed 2 November 2020).
Deeks 2021
-
- Deeks JJ, Higgins JP, Altman DG, editor(s). Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions. Version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook.
EndNote X9 [Computer program]
-
- EndNote X9. Clarivate, 2020.
GRADE pro GDT [Computer program]
-
- McMaster University (developed by Evidence Prime) GRADEpro GDT. Version accessed prior to July 2021. Hamilton (ON): McMaster University (developed by Evidence Prime). Available at gradepro.org.
Higgins 2003
Higgins 2019
-
- Higgins JP, Savovic J, Page MJ, Sterne JA. Revised Cochrane risk-of-bias tool for randomized trials (RoB 2). www.riskofbias.info/welcome/rob-2-0-tool/current-version-of-rob-2 (accessed 7 July 2021).
Higgins 2021a
-
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version version 6.2 (updated February 2021). Cochrane, 2021. Available from: training.cochrane.org/handbook 2020.
Higgins 2021b
-
- Higgins JP, Savović J, Page MJ, Elbers RG, Sterne JA. Chapter 8: Assessing risk of bias in a randomized trial. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook.
Higgins 2021c
-
- Higgins JP, Li T, Deeks JJ, editor(s). Chapter 6: Choosing effect measures and computing estimates of effect. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook.
Huang 2020
Johns Hopkins University 2021
-
- Johns Hopkins University. Mortality analyses. coronavirus.jhu.edu/data/mortality (accessed 11 March 2021).
Kreuzberger 2021
Lauer 2020
Li 2021
-
- Li T, Higgins JP, Deeks JJ (editors). Chapter 5: Collecting data. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions. Version 6.1 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook.
Liang 2020
Living Evidence Network 2019
-
- Living Evidence Network. Guidance for the production and publication of Cochrane living systematic reviews: Cochrane Reviews in living mode (Version December 2019). Available from community.cochrane.org/review-production/production-resources/living-sys... (accessed 22 March 2021).
Marshall 2020
Microsoft 2018 [Computer program]
-
- Microsoft Excel. Microsoft Corporation. Microsoft Corporation, 2018. office.microsoft.com/excel.
Moher 2009
-
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Journal of Clinical Epidemiology 2009;62(10):1006-12. - PubMed
Park 2020
Parmar 1998
-
- Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Statistics in Medicine 1998;17(24):2815-34. - PubMed
Pasin 2021
-
- Pasin L, Navalesi P, Zangrillo A, Kuzovlev A, Likhvantsev V, Hajjar LA, et al. Corticosteroids for patients with coronavirus disease 2019 (COVID-19) with different disease severity: a meta-analysis of randomized clinical trials. Journal of Cardiothoracic and Vascular Anesthesia 2021;35(2):578-84. [DOI: ] - PMC - PubMed
Piechotta 2021
-
- Piechotta V, Iannizzi C, Chai KL, Valk SJ, Kimber C, Dorando E, et al. Convalescent plasma or hyperimmune immunoglobulin for people with COVID‐19: a living systematic review. Cochrane Database of Systematic Reviews 2021, Issue 5. Art. No: CD013600. [DOI: 10.1002/14651858.CD013600.pub4] - DOI - PMC - PubMed
Ranganathan 2015
RevMan Web 2019 [Computer program]
-
- The Cochrane Collaboration Review Manager Web (RevMan Web). The Cochrane Collaboration, 2019. revman.cochrane.org.
Rhen 2005
Rochwerg 2018
-
- Rochwerg B, Oczkowski SJ, Siemieniuk RA, Agoritsas T, Belley-Cote E, D'Aragon F, et al. Corticosteroids in sepsis: an updated systematic review and meta-analysis. Critical Care Medicine 2018;46(9):1411-20. [PMID: ] - PubMed
Santesso 2020
-
- Santesso N, Glenton C, Dahm P, Garner P, Akl A, Alper B, et al. GRADE guidelines 26: informative statements to communicate the findings of systematic reviews of interventions. Journal of Clinical Epidemiology 2020;119:126-35. - PubMed
Schulte‐Schrepping 2020
Schünemann 2021
-
- Schünemann HJ, Higgins JP, Vist GE, Glasziou P, Akl EA, Skoetz N, et al. Chapter 14: Completing ‘Summary of findings’ tables and grading the certainty of the evidence. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021). Cochrane, 2021. Available from www.training.cochrane.org/handbook.
Siemieniuk 2020
-
- Siemieniuk RA, Bartoszko JJ, Ge L, Zeraatkar D, Izcovich A, Kum E, et al. Drug treatments for COVID-19: living systematic review and network meta-analysis. BMJ 2020;370:m2980. [BMJ: https://www.bmj.com/content/370/bmj.m2980] - PMC - PubMed
Skoetz 2020
-
- Skoetz N, Goldkuhle M, Van Dalen EC, Akl EA, Trivella M, Mustafa RA, et al. GRADE guidelines 27: how to calculate absolute effects for time-to-event outcomes in summary of findings tables and evidence profiles. Journal of Clinical Epidemiology 2020;118:124-31. - PubMed
Sterne 2019
-
- Sterne JA, Savovic J, Page MJ, Elbers RG, Blencowe N, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 2019;366:l4898. - PubMed
Sterne 2020
Stoelting 2006
-
- Stoelting RK, Hiller SC (editors). Chapter Pharmacology. In: Pharmacology & Physiology in Anesthetic Practice. 4 edition. Philadelphia: Lippincott Williams & Wilkins, 2006:462.
Struyf 2020
-
- Struyf T, Deeks JJ, Dinnes J, Takwoingi Y, Davenport C, Leeflang MM, et al. Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19 disease. Cochrane Database of Systematic Reviews 2020, Issue 7. Art. No: CD013665. [DOI: 10.1002/14651858.CD013665] - DOI - PMC - PubMed
Thibeault 2021
-
- Thibeault C, Mühlemann B, Helbig ET, Mittermaier M, Lingscheid T, Tober-Lau P, et al. Clinical and virological characteristics of hospitalised COVID-19 patients in a German tertiary care centre during the first wave of the SARS-CoV-2 pandemic: a prospective observational study. Infection 2021;-:1-12. - PMC - PubMed
Tierney 2007
Tong 2020
Van Paassen 2020
Villar 2020
-
- Villar J, Ferrando C, Martínez D, Ambrós A, Muñoz T, Soler JA, et al. Dexamethasone treatment for the acute respiratory distress syndrome: a multicentre, randomised controlled trial. Lancet Respiratory Medicine 2020;8(3):267-76. - PubMed
WHO 2007
-
- World Health Organization. Cumulative number of reported probable cases of SARS. www.who.int/csr/sars/country/2003_07_11/en (accessed 22 March 2021).
WHO 2019
-
- World Health Organization. Middle East respiratory syndrome coronavirus (MERS-CoV). www.who.int/emergencies/mers-cov/en (accessed 22 March 2021).
WHO 2020a
-
- World Health Organization. Report of the WHO‐China joint mission on coronavirus disease 2019 (COVID‐19). www.who.int/docs/default-source/coronaviruse/who-china-joint-mission-on-... (accessed 22 March 2021).
WHO 2020b
-
- World Health Organization. Estimating mortality from COVID-19 - scientific brief. www.who.int/publications/i/item/WHO-2019-nCoV-Sci-Brief-Mortality-2020.1 (accessed 22 March 2021).
WHO 2020c
WHO 2021a
-
- World Health Organization. WHO coronavirus disease (COVID-19) dashboard. covid19.who.int (accessed 1 March 2021).
WHO 2021b
-
- World Health Organization. Weekly epidemiological update - 23 February 2021. www.who.int/publications/m/item/weekly-epidemiological-update---23-febru... (accessed 1 March 2021).
WHO 2021c
-
- World Health Organization. SARS-CoV-2 variants. Available from www.who.int/csr/don/31-december-2020-sars-cov2-variants/en/ (accessed 22 March 2021).
WHO Living Guidance 2021
-
- World Health Organization 2021. COVID-19 Clinical management: living guidance. 6. Who do the recommendations apply to? www.who.int/publications/i/item/WHO-2019-nCoV-clinical-2021-1 2021;(3):14. [WHO WEBSITE: https://www.who.int/publications/i/item/WHO-2019-nCoV-therapeutics-2021.1]
WHO Living Guideline 2021
-
- WHO. Therapeutics and COVID-19: living guideline. Systemic corticosteroids. www.who.int/publications/i/item/WHO-2019-nCoV-therapeutics-2021.2;(3):37-50. [WHO WEBSITE: https://www.who.int/publications/i/item/WHO-2019-nCoV-therapeutics-2021.1]
Williamson 2020
World Bank Country Groups 2021
-
- World Bank Country and Lending Groups. datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-coun... (accessed 15 April 2021). [WEBSITE: https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-b...]
Wu 2020
References to other published versions of this review
Wagner 2021
-
- Wagner C, Griesel M, Micholajewska A, Fichtner F, Skoetz N. Corticosteroids for the treatment of COVID-19 (part of German Ecosystem CEO-Sys). PROSPERO 1 February 2021 (available from www.crd.york.ac.uk/PROSPERO/display_record.php?RecordID=227450). [PROSPERO: CRD42020227450]
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

