Glycerol suppresses glucose consumption in trypanosomes through metabolic contest
- PMID: 34388147
- PMCID: PMC8386887
- DOI: 10.1371/journal.pbio.3001359
Glycerol suppresses glucose consumption in trypanosomes through metabolic contest
Abstract
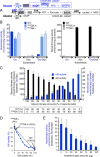
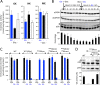
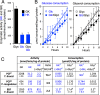
Microorganisms must make the right choice for nutrient consumption to adapt to their changing environment. As a consequence, bacteria and yeasts have developed regulatory mechanisms involving nutrient sensing and signaling, known as "catabolite repression," allowing redirection of cell metabolism to maximize the consumption of an energy-efficient carbon source. Here, we report a new mechanism named "metabolic contest" for regulating the use of carbon sources without nutrient sensing and signaling. Trypanosoma brucei is a unicellular eukaryote transmitted by tsetse flies and causing human African trypanosomiasis, or sleeping sickness. We showed that, in contrast to most microorganisms, the insect stages of this parasite developed a preference for glycerol over glucose, with glucose consumption beginning after the depletion of glycerol present in the medium. This "metabolic contest" depends on the combination of 3 conditions: (i) the sequestration of both metabolic pathways in the same subcellular compartment, here in the peroxisomal-related organelles named glycosomes; (ii) the competition for the same substrate, here ATP, with the first enzymatic step of the glycerol and glucose metabolic pathways both being ATP-dependent (glycerol kinase and hexokinase, respectively); and (iii) an unbalanced activity between the competing enzymes, here the glycerol kinase activity being approximately 80-fold higher than the hexokinase activity. As predicted by our model, an approximately 50-fold down-regulation of the GK expression abolished the preference for glycerol over glucose, with glucose and glycerol being metabolized concomitantly. In theory, a metabolic contest could be found in any organism provided that the 3 conditions listed above are met.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


Similar articles
-
Glycerol supports growth of the Trypanosoma brucei bloodstream forms in the absence of glucose: Analysis of metabolic adaptations on glycerol-rich conditions.PLoS Pathog. 2018 Nov 1;14(11):e1007412. doi: 10.1371/journal.ppat.1007412. eCollection 2018 Nov. PLoS Pathog. 2018. PMID: 30383867 Free PMC article.
-
The role of compartmentation and glycerol kinase in the synthesis of ATP within the glycosome of Trypanosoma brucei.J Biol Chem. 1985 Dec 15;260(29):15646-54. J Biol Chem. 1985. PMID: 2999127
-
Glycerol 3-phosphate alters Trypanosoma brucei hexokinase activity in response to environmental change.J Biol Chem. 2011 Sep 23;286(38):33150-7. doi: 10.1074/jbc.M111.235705. Epub 2011 Aug 3. J Biol Chem. 2011. PMID: 21813651 Free PMC article.
-
Glycosomes: A comprehensive view of their metabolic roles in T. brucei.Int J Biochem Cell Biol. 2017 Apr;85:85-90. doi: 10.1016/j.biocel.2017.01.015. Epub 2017 Feb 6. Int J Biochem Cell Biol. 2017. PMID: 28179189 Review.
-
Metabolic functions of glycosomes in trypanosomatids.Biochim Biophys Acta. 2006 Dec;1763(12):1463-77. doi: 10.1016/j.bbamcr.2006.08.019. Epub 2006 Aug 24. Biochim Biophys Acta. 2006. PMID: 17023066 Review.
Cited by
-
The bloodstream form of Trypanosoma brucei displays non-canonical gluconeogenesis.PLoS Negl Trop Dis. 2024 Feb 23;18(2):e0012007. doi: 10.1371/journal.pntd.0012007. eCollection 2024 Feb. PLoS Negl Trop Dis. 2024. PMID: 38394337 Free PMC article.
-
Fatty acid uptake in Trypanosoma brucei: Host resources and possible mechanisms.Front Cell Infect Microbiol. 2022 Nov 21;12:949409. doi: 10.3389/fcimb.2022.949409. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 36478671 Free PMC article. Review.
-
Glucose metabolism sustains heme-induced Trypanosoma cruzi epimastigote growth in vitro.PLoS Negl Trop Dis. 2023 Nov 10;17(11):e0011725. doi: 10.1371/journal.pntd.0011725. eCollection 2023 Nov. PLoS Negl Trop Dis. 2023. PMID: 37948458 Free PMC article.
-
Glycerol, a possible new player in the biology of trypanosomes.PLoS Pathog. 2021 Dec 2;17(12):e1010035. doi: 10.1371/journal.ppat.1010035. eCollection 2021 Dec. PLoS Pathog. 2021. PMID: 34855923 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

