SUMOylation of RepoMan during late telophase regulates dephosphorylation of lamin A
- PMID: 34387316
- PMCID: PMC8445599
- DOI: 10.1242/jcs.247171
SUMOylation of RepoMan during late telophase regulates dephosphorylation of lamin A
Abstract
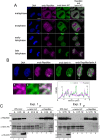
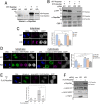
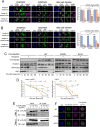
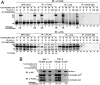
Dephosphorylation of lamin A, which triggers nuclear lamina reconstitution, is crucial for the completion of mitosis. However, the specific phosphatase and regulatory mechanism that allow timely lamin A dephosphorylation remain unclear. Here, we report that RepoMan (also known as CDCA2), a regulatory subunit of protein phosphatase 1γ (PP1γ) is transiently modified with SUMO-2 at K762 during late telophase. SUMOylation of RepoMan markedly enhanced its binding affinity with lamin A. Moreover, SUMOylated RepoMan contributes to lamin A recruitment to telophase chromosomes and dephosphorylation of the mitotic lamin A phosphorylation. Expression of a SUMO-2 mutant that has a defective interaction with the SUMO-interacting motif (SIM) resulted in failure of the lamin A and RepoMan association, along with abrogation of lamin A dephosphorylation and subsequent nuclear lamina formation. These findings strongly suggest that RepoMan recruits lamin A through SUMO-SIM interaction. Thus, transient SUMOylation of RepoMan plays an important role in the spatiotemporal regulation of lamin A dephosphorylation and the subsequent nuclear lamina formation at the end of mitosis.
Keywords: Lamin; Mitosis; Nuclear lamina; Protein phosphatase; SIM; SUMO-interacting motif; Sumoylation.
© 2021. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interests The authors declare no competing or financial interests.
Figures



Similar articles
-
Repo-Man/protein phosphatase 1 SUMOylation mediates binding to lamin A and serine 22 dephosphorylation.Open Biol. 2022 Apr;12(4):220017. doi: 10.1098/rsob.220017. Epub 2022 Apr 13. Open Biol. 2022. PMID: 35414260 Free PMC article.
-
Lamin A reassembly at the end of mitosis is regulated by its SUMO-interacting motif.Exp Cell Res. 2016 Mar 1;342(1):83-94. doi: 10.1016/j.yexcr.2016.02.016. Epub 2016 Feb 24. Exp Cell Res. 2016. PMID: 26921507
-
Emerin prevents BAF-mediated aggregation of lamin A on chromosomes in telophase to allow nuclear membrane expansion and nuclear lamina formation.Mol Biol Cell. 2022 Dec 1;33(14):ar137. doi: 10.1091/mbc.E22-01-0007. Epub 2022 Oct 6. Mol Biol Cell. 2022. PMID: 36200863 Free PMC article.
-
LEM4/ANKLE-2 deficiency impairs post-mitotic re-localization of BAF, LAP2α and LaminA to the nucleus, causes nuclear envelope instability in telophase and leads to hyperploidy in HeLa cells.Eur J Cell Biol. 2018 Jan;97(1):63-74. doi: 10.1016/j.ejcb.2017.12.001. Epub 2017 Dec 11. Eur J Cell Biol. 2018. PMID: 29254732
-
Emerin and the nuclear lamina in muscle and cardiac disease.Circ Res. 2008 Jul 3;103(1):16-23. doi: 10.1161/CIRCRESAHA.108.172197. Circ Res. 2008. PMID: 18596264 Review.
Cited by
-
Repo-Man/protein phosphatase 1 SUMOylation mediates binding to lamin A and serine 22 dephosphorylation.Open Biol. 2022 Apr;12(4):220017. doi: 10.1098/rsob.220017. Epub 2022 Apr 13. Open Biol. 2022. PMID: 35414260 Free PMC article.
-
SUMOylation at the inner nuclear membrane facilitates nuclear envelope biogenesis during mitosis.J Cell Biol. 2023 Aug 7;222(8):e202208137. doi: 10.1083/jcb.202208137. Epub 2023 Jul 3. J Cell Biol. 2023. PMID: 37398994 Free PMC article.
-
Dephosphorylation in nuclear reassembly after mitosis.Front Cell Dev Biol. 2022 Oct 4;10:1012768. doi: 10.3389/fcell.2022.1012768. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 36268509 Free PMC article. Review.
-
Sculpting nuclear envelope identity from the endoplasmic reticulum during the cell cycle.Nucleus. 2024 Dec;15(1):2299632. doi: 10.1080/19491034.2023.2299632. Epub 2024 Jan 18. Nucleus. 2024. PMID: 38238284 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

