The influence of the gut microbiota on the bioavailability of oral drugs
- PMID: 34386321
- PMCID: PMC8343123
- DOI: 10.1016/j.apsb.2020.09.013
The influence of the gut microbiota on the bioavailability of oral drugs
Abstract
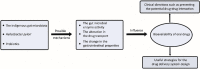
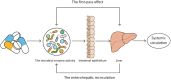
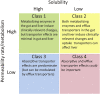
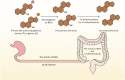
Due to its safety, convenience, low cost and good compliance, oral administration attracts lots of attention. However, the efficacy of many oral drugs is limited to their unsatisfactory bioavailability in the gastrointestinal tract. One of the critical and most overlooked factors is the symbiotic gut microbiota that can modulate the bioavailability of oral drugs by participating in the biotransformation of oral drugs, influencing the drug transport process and altering some gastrointestinal properties. In this review, we summarized the existing research investigating the possible relationship between the gut microbiota and the bioavailability of oral drugs, which may provide great ideas and useful instructions for the design of novel drug delivery systems or the achievement of personalized medicine.
Keywords: 5-ASA, 5-aminosalicylic acid; AA, ascorbic acid; ABC, ATP-binding cassette; ACS, amphipathic chitosan derivative; AMI, amiodarone; AQP4, aquaporin 4; AR, azoreductase; ASP, amisulpride; BBR, berberine; BCRP, breast cancer resistance protein; BCS, biopharmaceutics classification system; BDDCS, the biopharmaceutics drug disposition classification system; BDEPT, the bacteria-directed enzyme prodrug therapy; BSH, bile salt hydrolase; Bioavailability; CA, cholic acid; CDCA, chenodeoxycholic acid; CPP, cell-penetrating peptide; CS, chitosan; Colon-specific drug delivery system; DCA, deoxycholic acid; DRPs, digoxin reduction products; EcN, Escherichia coli Nissle 1917; FA, folate; FAO, Food and Agriculture Organization of the United Nations; GCDC, glycochenodeoxycholate; GL, glycyrrhizic acid; Gut microbiota; HFD, high fat diet; HTC, hematocrit; IBD, inflammatory bowel disease; LCA, lithocholic acid; LPS, lipopolysaccharide; MATEs, multidrug and toxin extrusion proteins; MDR1, multidrug resistance gene 1; MDR1a, multidrug resistance protein-1a; MKC, monoketocholic acid; MPA, mycophenolic acid; MRP2, multidrug resistance-associated protein 2; NEC, necrotizing enterocolitis; NMEs, new molecular entities; NRs, nitroreductases; NSAIDs, non-steroidal anti-inflammatory drugs; NaDC, sodium deoxycholate; NaGC, sodium glycholate; OATs, organic anion transporters; OCTNs, organic zwitterion/cation; OCTs, organic cation transporters; Oral drugs; P-gp, P-glycoprotein; PD, Parkinson's disease; PPIs, proton pump inhibitors; PT, pectin; PWSDs, poorly water-soluble drugs; Probiotics; RA, rheumatoid arthritis; RBC, red blood cell; SCFAs, short-chain fatty acids; SGLT-1, sodium-coupled glucose transporter 1; SLC, solute carrier; SLN, solid lipid nanoparticle; SP, sulfapyridine; SSZ, sulfasalazine; SVCT-1/2, the sodium-dependent vitamin C transporter-1/2; T1D, type 1 diabetes; T1DM, type 1 diabetes mellitus; T2D, type 2 diabetes; TCA, taurocholate; TCDC, taurochenodeoxycholate; TDCA, taurodeoxycholate; TLCA, taurolithocholate; TME, the tumor microenvironment; UDC, ursodeoxycholic acid; WHO, World Health Organization; an OTC drug, an over-the-counter drug; cgr operon, cardiac glycoside reductase operon; dhBBR, dihydroberberine; pKa, dissociation constant; the GI tract, the gastrointestinal tract.
© 2021 Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences. Production and hosting by Elsevier B.V.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures


Similar articles
-
Understanding peroral absorption: regulatory aspects and contemporary approaches to tackling solubility and permeability hurdles.Acta Pharm Sin B. 2017 May;7(3):260-280. doi: 10.1016/j.apsb.2016.09.005. Epub 2016 Nov 2. Acta Pharm Sin B. 2017. PMID: 28540164 Free PMC article. Review.
-
Bile acid nuclear receptor FXR and digestive system diseases.Acta Pharm Sin B. 2015 Mar;5(2):135-44. doi: 10.1016/j.apsb.2015.01.004. Epub 2015 Feb 25. Acta Pharm Sin B. 2015. PMID: 26579439 Free PMC article. Review.
-
Gut Microbiota-Mediated Bile Acid Transformations Alter the Cellular Response to Multidrug Resistant Transporter Substrates in Vitro: Focus on P-glycoprotein.Mol Pharm. 2018 Dec 3;15(12):5711-5727. doi: 10.1021/acs.molpharmaceut.8b00875. Epub 2018 Nov 16. Mol Pharm. 2018. PMID: 30388019
-
Transporter-Mediated Drug-Drug Interactions and Their Significance.Adv Exp Med Biol. 2019;1141:241-291. doi: 10.1007/978-981-13-7647-4_5. Adv Exp Med Biol. 2019. PMID: 31571167 Review.
-
Bile acid coordinates microbiota homeostasis and systemic immunometabolism in cardiometabolic diseases.Acta Pharm Sin B. 2022 May;12(5):2129-2149. doi: 10.1016/j.apsb.2021.12.011. Epub 2021 Dec 22. Acta Pharm Sin B. 2022. PMID: 35646540 Free PMC article. Review.
Cited by
-
Morinda officinalis oligosaccharides increase serotonin in the brain and ameliorate depression via promoting 5-hydroxytryptophan production in the gut microbiota.Acta Pharm Sin B. 2022 Aug;12(8):3298-3312. doi: 10.1016/j.apsb.2022.02.032. Epub 2022 Mar 3. Acta Pharm Sin B. 2022. PMID: 35967282 Free PMC article.
-
Metabolites Analysis of Anti-Myocardial Ischemia Active Components of Saussurea involucrata Based on Gut Microbiota-Drug Interaction.Int J Mol Sci. 2022 Jul 5;23(13):7457. doi: 10.3390/ijms23137457. Int J Mol Sci. 2022. PMID: 35806462 Free PMC article.
-
Challenges and Opportunities in the Oral Delivery of Recombinant Biologics.Pharmaceutics. 2023 May 5;15(5):1415. doi: 10.3390/pharmaceutics15051415. Pharmaceutics. 2023. PMID: 37242657 Free PMC article. Review.
-
Are Local Drug Delivery Systems a Challenge in Clinical Periodontology?J Clin Med. 2023 Jun 19;12(12):4137. doi: 10.3390/jcm12124137. J Clin Med. 2023. PMID: 37373830 Free PMC article. Review.
-
Nanobiotechnology boosts ferroptosis: opportunities and challenges.J Nanobiotechnology. 2024 Oct 8;22(1):606. doi: 10.1186/s12951-024-02842-5. J Nanobiotechnology. 2024. PMID: 39379969 Free PMC article. Review.
References
-
- Enright E.F., Griffin B.T., Gahan C.G.M., Joyce S.A. Microbiome-mediated bile acid modification: role in intestinal drug absorption and metabolism. Pharmacol Res. 2018;133:170–186. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

