Obstructive Sleep Apnea Is Associated With Low Testosterone Levels in Severely Obese Men
- PMID: 34381420
- PMCID: PMC8350060
- DOI: 10.3389/fendo.2021.622496
Obstructive Sleep Apnea Is Associated With Low Testosterone Levels in Severely Obese Men
Abstract
Background: Disrupted sleep affects cardio-metabolic and reproductive health. Obstructive sleep apnea syndrome represents a major complication of obesity and has been associated with gonadal axis activity changes and lower serum testosterone concentration in men. However, there is no consistent opinion on the effect of obstructive sleep apnea on testosterone levels in men.
Objective: The aim of this study was to determine the influence of obstructive sleep apnea on total and free testosterone levels in severely obese men.
Materials and methods: The study included 104 severely obese (Body Mass Index (BMI) ≥ 35 kg/m2) men, aged 20 to 60, who underwent anthropometric, blood pressure, fasting plasma glucose, lipid profile, and sex hormone measurements. All participants were subjected to polysomnography. According to apnea-hypopnea index (AHI) patients were divided into 3 groups: <15 (n = 20), 15 - 29.9 (n = 17) and ≥ 30 (n = 67).
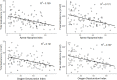
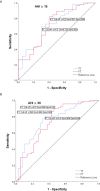
Results: There was a significant difference between AHI groups in age (29.1 ± 7.2, 43.2 ± 13.2, 45.2 ± 10.2 years; p < 0.001), BMI (42.8 ± 5.9, 43.2 ± 5.9, 47.1 ± 7.8 kg/m2; p = 0.023), the prevalence of metabolic syndrome (MetS) (55%, 82.4%, 83.6%, p = 0.017), continuous metabolic syndrome score (siMS) (4.01 ± 1.21, 3.42 ± 0.80, 3.94 ± 1.81, 4.20 ± 1.07; p = 0.038), total testosterone (TT) (16.6 ± 6.1, 15.2 ± 5.3, 11.3 ± 4.44 nmol/l; p < 0.001) and free testosterone (FT) levels (440.4 ± 160.8, 389.6 ± 162.5, 294.5 ± 107.0 pmol/l; p < 0.001). TT level was in a significant negative correlation with AHI, oxygen desaturation index (ODI), BMI, MetS and siMS. Also, FT was in a significant negative correlation with AHI, ODI, BMI, age, MetS and siMS. The multiple regression analysis revealed that both AHI and ODI were in significant correlation with TT and FT after adjustment for age, BMI, siMS score and MetS components.
Conclusion: Obstructive sleep apnea is associated with low TT and FT levels in severely obese men.
Keywords: male; metabolic syndrome; obesity; sleep apnea; testosterone.
Copyright © 2021 Tančić-Gajić, Vukčević, Ivović, Marina, Arizanović, Soldatović, Stojanović, Đogo, Kendereški and Vujović.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Comment in
-
Benign Prostatic Hyperplasia.J Urol. 2022 Jan;207(1):201-204. doi: 10.1097/JU.0000000000002275. Epub 2021 Oct 18. J Urol. 2022. PMID: 34661444 No abstract available.
Similar articles
-
Testosterone levels in obese male patients with obstructive sleep apnea syndrome: relation to oxygen desaturation, body weight, fat distribution and the metabolic parameters.J Endocrinol Invest. 2003 Jun;26(6):493-8. doi: 10.1007/BF03345209. J Endocrinol Invest. 2003. PMID: 12952360
-
[Metabolic profile in obese patients with obstructive sleep apnea. A comparison between patients with insulin resistance and with insulin sensitivity].Pneumologia. 2014 Apr-Jun;63(2):100-2, 104-6. Pneumologia. 2014. PMID: 25241557 Romanian.
-
Nocturnal Hypoxemia is Associated With Low Testosterone Levels in Overweight Males and Older Men With Normal Weight.J Clin Sleep Med. 2017 Dec 15;13(12):1395-1401. doi: 10.5664/jcsm.6832. J Clin Sleep Med. 2017. PMID: 29065959 Free PMC article.
-
Obstructive sleep apnea is associated with increased arterial stiffness in severe obesity.J Sleep Res. 2014 Dec;23(6):700-708. doi: 10.1111/jsr.12156. Epub 2014 Apr 15. J Sleep Res. 2014. PMID: 24731017
-
Impact of OSA on biological markers in morbid obesity and metabolic syndrome.J Clin Sleep Med. 2014 Mar 15;10(3):263-70. doi: 10.5664/jcsm.3524. J Clin Sleep Med. 2014. PMID: 24634623 Free PMC article.
Cited by
-
Multifactorial sleep disturbance in Klinefelter syndrome: a case report.Transl Androl Urol. 2023 Jul 31;12(7):1204-1210. doi: 10.21037/tau-22-587. Epub 2023 Jul 3. Transl Androl Urol. 2023. PMID: 37554521 Free PMC article.
-
The complex relation between obstructive sleep apnoea syndrome, hypogonadism and testosterone replacement therapy.Front Reprod Health. 2023 Oct 10;5:1219239. doi: 10.3389/frph.2023.1219239. eCollection 2023. Front Reprod Health. 2023. PMID: 37881222 Free PMC article. Review.
-
Chronic intermittent hypoxia-induced hypertension: the impact of sex hormones.Am J Physiol Regul Integr Comp Physiol. 2024 May 1;326(5):R333-R345. doi: 10.1152/ajpregu.00258.2023. Epub 2024 Feb 26. Am J Physiol Regul Integr Comp Physiol. 2024. PMID: 38406843 Free PMC article. Review.
-
Effect of obstructive sleep apnea on semen quality.Sleep Breath. 2023 Dec;27(6):2341-2349. doi: 10.1007/s11325-023-02847-8. Epub 2023 May 15. Sleep Breath. 2023. PMID: 37184755
-
Effects of very low-calorie ketogenic diet on hypothalamic-pituitary-adrenal axis and renin-angiotensin-aldosterone system.J Endocrinol Invest. 2023 Aug;46(8):1509-1520. doi: 10.1007/s40618-023-02068-6. Epub 2023 Apr 5. J Endocrinol Invest. 2023. PMID: 37017918 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

