FcRn-Targeted Mucosal Vaccination against Influenza Virus Infection
- PMID: 34380652
- PMCID: PMC8387404
- DOI: 10.4049/jimmunol.2100297
FcRn-Targeted Mucosal Vaccination against Influenza Virus Infection
Abstract
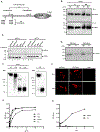
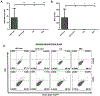
The respiratory tract is constantly exposed to various airborne pathogens. Most vaccines against respiratory infections are designed for the parenteral routes of administration; consequently, they provide relatively minimal protection in the respiratory tract. A vaccination strategy that aims to induce the protective mucosal immune responses in the airway is urgently needed. The FcRn mediates IgG Ab transport across the epithelial cells lining the respiratory tract. By mimicking this natural IgG transfer, we tested whether FcRn delivers vaccine Ags to induce a protective immunity to respiratory infections. In this study, we designed a monomeric IgG Fc fused to influenza virus hemagglutinin (HA) Ag with a trimerization domain. The soluble trimeric HA-Fc were characterized by their binding with conformation-dependent HA Abs or FcRn. In wild-type, but not FcRn knockout, mice, intranasal immunization with HA-Fc plus CpG adjuvant conferred significant protection against lethal intranasal challenge with influenza A/PR/8/34 virus. Further, mice immunized with a mutant HA-Fc lacking FcRn binding sites or HA alone succumbed to lethal infection. Protection was attributed to high levels of neutralizing Abs, robust and long-lasting B and T cell responses, the presence of lung-resident memory T cells and bone marrow plasma cells, and a remarkable reduction of virus-induced lung inflammation. Our results demonstrate for the first time, to our knowledge, that FcRn can effectively deliver a trimeric viral vaccine Ag in the respiratory tract and elicit potent protection against respiratory infection. This study further supports a view that FcRn-mediated mucosal immunization is a platform for vaccine delivery against common respiratory pathogens.
Copyright © 2021 by The American Association of Immunologists, Inc.
Figures
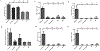
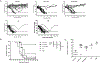

Similar articles
-
Intranasal vaccination of recombinant H5N1 HA1 proteins fused with foldon and Fc induces strong mucosal immune responses with neutralizing activity: Implication for developing novel mucosal influenza vaccines.Hum Vaccin Immunother. 2015;11(12):2831-8. doi: 10.1080/21645515.2015.1074363. Hum Vaccin Immunother. 2015. PMID: 26260706 Free PMC article.
-
Intranasal adenovirus-vectored vaccine for induction of long-lasting humoral immunity-mediated broad protection against influenza in mice.J Virol. 2014 Sep 1;88(17):9693-703. doi: 10.1128/JVI.00823-14. Epub 2014 Jun 11. J Virol. 2014. PMID: 24920793 Free PMC article.
-
The Potential of Neuraminidase as an Antigen for Nasal Vaccines To Increase Cross-Protection against Influenza Viruses.J Virol. 2021 Sep 27;95(20):e0118021. doi: 10.1128/JVI.01180-21. Epub 2021 Aug 11. J Virol. 2021. PMID: 34379511 Free PMC article.
-
Intranasal Inactivated Influenza Vaccines: a Reasonable Approach to Improve the Efficacy of Influenza Vaccine?Jpn J Infect Dis. 2016;69(3):165-79. doi: 10.7883/yoken.JJID.2015.560. Jpn J Infect Dis. 2016. PMID: 27212584 Review.
-
Defense mechanisms against influenza virus infection in the respiratory tract mucosa.Jpn J Infect Dis. 2004 Dec;57(6):236-47. Jpn J Infect Dis. 2004. PMID: 15623947 Review.
Cited by
-
Olfactory immunology: the missing piece in airway and CNS defence.Nat Rev Immunol. 2024 Jun;24(6):381-398. doi: 10.1038/s41577-023-00972-9. Epub 2023 Dec 14. Nat Rev Immunol. 2024. PMID: 38097777 Free PMC article. Review.
-
The therapeutic age of the neonatal Fc receptor.Nat Rev Immunol. 2023 Jul;23(7):415-432. doi: 10.1038/s41577-022-00821-1. Epub 2023 Feb 1. Nat Rev Immunol. 2023. PMID: 36726033 Free PMC article. Review.
-
An FcRn-targeted mucosal vaccine against SARS-CoV-2 infection and transmission.Nat Commun. 2023 Nov 6;14(1):7114. doi: 10.1038/s41467-023-42796-0. Nat Commun. 2023. PMID: 37932271 Free PMC article.
-
Harnessing the potential of the NALT and BALT as targets for immunomodulation using engineering strategies to enhance mucosal uptake.Front Immunol. 2024 Sep 2;15:1419527. doi: 10.3389/fimmu.2024.1419527. eCollection 2024. Front Immunol. 2024. PMID: 39286244 Free PMC article. Review.
-
A broadly applicable protein-polymer adjuvant system for antiviral vaccines.EMBO Mol Med. 2024 Jun;16(6):1451-1483. doi: 10.1038/s44321-024-00076-4. Epub 2024 May 15. EMBO Mol Med. 2024. PMID: 38750307 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

