Medicated Hydroxyapatite/Collagen Hybrid Scaffolds for Bone Regeneration and Local Antimicrobial Therapy to Prevent Bone Infections
- PMID: 34371782
- PMCID: PMC8309148
- DOI: 10.3390/pharmaceutics13071090
Medicated Hydroxyapatite/Collagen Hybrid Scaffolds for Bone Regeneration and Local Antimicrobial Therapy to Prevent Bone Infections
Abstract
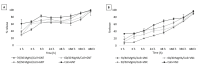
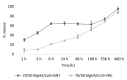
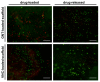
Microbial infections occurring during bone surgical treatment, the cause of osteomyelitis and implant failures, are still an open challenge in orthopedics. Conventional therapies are often ineffective and associated with serious side effects due to the amount of drugs administered by systemic routes. In this study, a medicated osteoinductive and bioresorbable bone graft was designed and investigated for its ability to control antibiotic drug release in situ. This represents an ideal solution for the eradication or prevention of infection, while simultaneously repairing bone defects. Vancomycin hydrochloride and gentamicin sulfate, here considered for testing, were loaded into a previously developed and largely investigated hybrid bone-mimetic scaffold made of collagen fibers biomineralized with magnesium doped-hydroxyapatite (MgHA/Coll), which in the last ten years has widely demonstrated its effective potential in bone tissue regeneration. Here, we have explored whether it can be used as a controlled local delivery system for antibiotic drugs. An easy loading method was selected in order to be reproducible, quickly, in the operating room. The maintenance of the antibacterial efficiency of the released drugs and the biosafety of medicated scaffolds were assessed with microbiological and in vitro tests, which demonstrated that the MgHA/Coll scaffolds were safe and effective as a local delivery system for an extended duration therapy-promising results for the prevention of bone defect-related infections in orthopedic surgeries.
Keywords: biomimetic materials; bone regeneration; gentamicin sulfate; local therapy; microbial bone infection; vancomycin hydrochloride.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
In vitro and in vivo osteogenic activity of the novel vancomycin-loaded bone-like hydroxyapatite/poly(amino acid) scaffold.J Biomater Appl. 2016 May;30(10):1566-77. doi: 10.1177/0885328215623735. Epub 2015 Dec 18. J Biomater Appl. 2016. PMID: 26686585
-
Release of Antibiotics Out of a Moldable Collagen-β-Tricalciumphosphate-Composite Compared to Two Calcium Phosphate Granules.Materials (Basel). 2019 Dec 5;12(24):4056. doi: 10.3390/ma12244056. Materials (Basel). 2019. PMID: 31817409 Free PMC article.
-
In vitro and in vivo drug release and antibacterial properties of the novel vancomycin-loaded bone-like hydroxyapatite/poly amino acid scaffold.Int J Nanomedicine. 2017 Mar 8;12:1841-1851. doi: 10.2147/IJN.S122864. eCollection 2017. Int J Nanomedicine. 2017. PMID: 28331309 Free PMC article.
-
Biodegradable Scaffolds for Bone Regeneration Combined with Drug-Delivery Systems in Osteomyelitis Therapy.Pharmaceuticals (Basel). 2017 Dec 12;10(4):96. doi: 10.3390/ph10040096. Pharmaceuticals (Basel). 2017. PMID: 29231857 Free PMC article. Review.
-
Bone infections and bone graft substitutes for local antibiotic therapy.Surg Technol Int. 2014 Mar;24:353-62. Surg Technol Int. 2014. PMID: 24504740 Review.
Cited by
-
Complex Evaluation of Nanocomposite-Based Hydroxyapatite for Biomedical Applications.Biomimetics (Basel). 2023 Nov 6;8(7):528. doi: 10.3390/biomimetics8070528. Biomimetics (Basel). 2023. PMID: 37999169 Free PMC article.
-
Addressing the Needs of the Rapidly Aging Society through the Development of Multifunctional Bioactive Coatings for Orthopedic Applications.Int J Mol Sci. 2022 Mar 3;23(5):2786. doi: 10.3390/ijms23052786. Int J Mol Sci. 2022. PMID: 35269928 Free PMC article. Review.
-
Functionalization of biomimetic mineralized collagen for bone tissue engineering.Mater Today Bio. 2023 May 6;20:100660. doi: 10.1016/j.mtbio.2023.100660. eCollection 2023 Jun. Mater Today Bio. 2023. PMID: 37214545 Free PMC article. Review.
-
Collagen-hydroxyapatite based scaffolds for bone trauma and regeneration: recent trends and future perspectives.Nanomedicine (Lond). 2024;19(18-20):1689-1709. doi: 10.1080/17435889.2024.2375958. Epub 2024 Aug 20. Nanomedicine (Lond). 2024. PMID: 39163266 Review.
-
Biocompatibility Studies on a Collagen-Hydroxyapatite Biomaterial.Curr Health Sci J. 2022 Apr-Jun;48(2):217-225. doi: 10.12865/CHSJ.48.02.12. Epub 2022 Jun 30. Curr Health Sci J. 2022. PMID: 36320879 Free PMC article.
References
-
- Tampieri A., Sandri M., Landi E., Sprio S., Valentini F., Boskey A. Synthetic biomineralisation yielding HA/collagen hybrid composite. Adv. Appl. Ceram. 2008;107:298–302. doi: 10.1179/174367608X314163. - DOI
LinkOut - more resources
Full Text Sources

