Lipid Nanoparticles Traverse Non-Corneal Path to Reach the Posterior Eye Segment: In Vivo Evidence
- PMID: 34361825
- PMCID: PMC8347557
- DOI: 10.3390/molecules26154673
Lipid Nanoparticles Traverse Non-Corneal Path to Reach the Posterior Eye Segment: In Vivo Evidence
Abstract
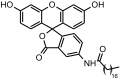
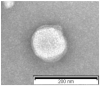
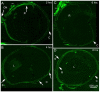
Lipid-based nanocarriers (LNs) have made it possible to prolong corneal residence time and improve the ocular bioavailability of ophthalmic drugs. In order to investigate how the LNs interact with the ocular mucosa and reach the posterior eye segment, we have formulated lipid nanocarriers that were designed to bear a traceable fluorescent probe in the present work. The chosen fluorescent probe was obtained by a conjugation reaction between fluoresceinamine and the solid lipid excipient stearic acid, forming a chemically synthesized adduct (ODAF, N-(3',6'-dihydroxy-3-oxospiro [isobenzofuran-1(3H),9'-[9H] xanthen]-5-yl)-octadecanamide). The novel formulation (LN-ODAF) has been formulated and characterized in terms of its technological parameters (polydispersity index, mean particle size and zeta potential), while an in vivo study was carried out to assess the ability of LN-ODAF to diffuse through different ocular compartments. LN-ODAF were in nanometric range (112.7 nm ± 0.4), showing a good homogeneity and long-term stability. A TEM (transmission electron microscopy) study corroborated these results of characterization. In vivo results pointed out that after ocular instillation, LN ODAF were concentrated in the cornea (two hours), while at a longer time (from the second hour to the eighth hour), the fluorescent signals extended gradually towards the back of the eye. From the results obtained, LN-ODAF demonstrated a potential use of lipid-based nanoparticles as efficient carriers of an active pharmaceutical ingredient (API) involved in the management of retinal diseases.
Keywords: fluorescence microscopy; fluorescent nanoparticle; nanomedicine; ocular drug delivery; retina.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures
Similar articles
-
Solid lipid nanoparticles as promising tool for intraocular tobramycin delivery: Pharmacokinetic studies on rabbits.Eur J Pharm Biopharm. 2016 Dec;109:214-223. doi: 10.1016/j.ejpb.2016.10.006. Epub 2016 Oct 24. Eur J Pharm Biopharm. 2016. PMID: 27789355
-
Lipo-PEG nano-ocular formulation successfully encapsulates hydrophilic fluconazole and traverses corneal and non-corneal path to reach posterior eye segment.J Drug Target. 2021 Jul;29(6):631-650. doi: 10.1080/1061186X.2020.1871483. Epub 2021 Feb 1. J Drug Target. 2021. PMID: 33410357
-
Fabrication and evaluation of lipid nanoparticulates for ocular delivery of a COX-2 inhibitor.Drug Deliv. 2016 Nov;23(9):3364-3373. doi: 10.1080/10717544.2016.1183720. Epub 2016 May 17. Drug Deliv. 2016. PMID: 27128623
-
Application of lipid nanoparticles to ocular drug delivery.Expert Opin Drug Deliv. 2016 Dec;13(12):1743-1757. doi: 10.1080/17425247.2016.1201059. Epub 2016 Jun 24. Expert Opin Drug Deliv. 2016. PMID: 27291069 Review.
-
Lipid nanoparticles as drug/gene delivery systems to the retina.J Ocul Pharmacol Ther. 2013 Mar;29(2):173-88. doi: 10.1089/jop.2012.0128. Epub 2013 Jan 3. J Ocul Pharmacol Ther. 2013. PMID: 23286300 Review.
Cited by
-
Corneal wound healing and nerve regeneration by novel ophthalmic formulations based on cross-linked sodium hyaluronate, taurine, vitamin B6, and vitamin B12.Front Pharmacol. 2023 Feb 2;14:1109291. doi: 10.3389/fphar.2023.1109291. eCollection 2023. Front Pharmacol. 2023. PMID: 36817120 Free PMC article.
-
Hyaluronan-Cholesterol Nanogels for the Enhancement of the Ocular Delivery of Therapeutics.Pharmaceutics. 2021 Oct 25;13(11):1781. doi: 10.3390/pharmaceutics13111781. Pharmaceutics. 2021. PMID: 34834195 Free PMC article.
-
Cellulosic Polymers for Enhancing Drug Bioavailability in Ocular Drug Delivery Systems.Pharmaceuticals (Basel). 2021 Nov 22;14(11):1201. doi: 10.3390/ph14111201. Pharmaceuticals (Basel). 2021. PMID: 34832983 Free PMC article. Review.
-
Fluoxetine Protects Retinal Ischemic Damage in Mice.Pharmaceutics. 2023 Apr 29;15(5):1370. doi: 10.3390/pharmaceutics15051370. Pharmaceutics. 2023. PMID: 37242611 Free PMC article.
-
Development of Solid Lipid Nanoparticles as Dry Powder: Characterization and Formulation Considerations.Molecules. 2023 Feb 5;28(4):1545. doi: 10.3390/molecules28041545. Molecules. 2023. PMID: 36838532 Free PMC article.
References
-
- de Oliveira I.F., Barbosa E.J., Peters M.C.C., Henostroza M.A.B., Yukuyama M.N., Dos Santos Neto E., Löbenberg R., Bou-Chacra N. Cutting-edge advances in therapy for the posterior segment of the eye: Solid lipid nanoparticles and nanostructured lipid carriers. Int. J. Pharm. 2020;589:119831. doi: 10.1016/j.ijpharm.2020.119831. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

