Identification of Spiro-Fused [3-azabicyclo[3.1.0]hexane]oxindoles as Potential Antitumor Agents: Initial In Vitro Evaluation of Anti-Proliferative Effect and Actin Cytoskeleton Transformation in 3T3 and 3T3-SV40 Fibroblast
- PMID: 34361029
- PMCID: PMC8347490
- DOI: 10.3390/ijms22158264
Identification of Spiro-Fused [3-azabicyclo[3.1.0]hexane]oxindoles as Potential Antitumor Agents: Initial In Vitro Evaluation of Anti-Proliferative Effect and Actin Cytoskeleton Transformation in 3T3 and 3T3-SV40 Fibroblast
Abstract
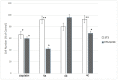
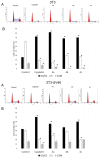
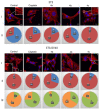
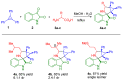
Novel heterocyclic compounds containing 3-spiro[3-azabicyclo[3.1.0]hexane]oxindole framework (4a, 4b and 4c) have been studied as potential antitumor agents. The in silico ADMET (adsorption, distribution, metabolism, excretion and toxicity) analysis was performed on 4a-c compounds with promising antiproliferative activity, previously synthetized and screened against human erythroleukemic cell line K562 tumor cell line. Cytotoxicity of 4a-c against murine fibroblast 3T3 and SV-40 transformed murine fibroblast 3T3-SV40 cell lines were evaluated. The 4a and 4c compounds were cytotoxic against 3T3-SV40 cells in comparison with those of 3T3. In agreement with the DNA cytometry studies, the tested compounds have achieved significant cell-cycle perturbation with higher accumulation of cells in G0/G1 phase. Using confocal microscopy, we found that with 4a and 4c treatment of 3T3 cells, actin filaments disappeared, and granular actin was distributed diffusely in the cytoplasm in 82-97% of cells. The number of 3T3-SV40 cells with stress fibers increased to 7-30% against 2% in control. We discovered that transformed 3T3-SV40 cells after treatment with compounds 4a and 4c significantly reduced the number of cells with filopodium-like membrane protrusions (from 86 % in control cells to 6-18% after treatment), which indirectly suggests a decrease in cell motility. We can conclude that the studied compounds 4a and 4c have a cytostatic effect, which can lead to a decrease in the number of filopodium-like membrane protrusions.
Keywords: 3-spiro[azabicyclo[3.1.0]hexane]oxindoles; 3T3; 3T3-SV40; ADMET analysis; actin; cell cycle; cytoskeleton; metastasis; tumor cells.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Biological Evaluation of 3-Azaspiro[Bicyclo[3.1.0]Hexane-2,5'-Pyrimidines] as Potential Antitumor Agents.Int J Mol Sci. 2022 Sep 15;23(18):10759. doi: 10.3390/ijms231810759. Int J Mol Sci. 2022. PMID: 36142688 Free PMC article.
-
Identification of Spiro-Fused Pyrrolo[3,4-a]pyrrolizines and Tryptanthrines as Potential Antitumor Agents: Synthesis and In Vitro Evaluation.Int J Mol Sci. 2021 Nov 5;22(21):11997. doi: 10.3390/ijms222111997. Int J Mol Sci. 2021. PMID: 34769424 Free PMC article.
-
[Antioxidants-induced rearrangements of actin cytoskeleton in 3T3 and 3T3-SV40 fibroblasts].Tsitologiia. 2004;46(5):395-403. Tsitologiia. 2004. PMID: 15344883 Russian.
-
Spiro-oxindoles as a Promising Class of Small Molecule Inhibitors of p53-MDM2 Interaction Useful in Targeted Cancer Therapy.Top Curr Chem (Cham). 2017 Feb;375(1):3. doi: 10.1007/s41061-016-0089-0. Epub 2016 Dec 9. Top Curr Chem (Cham). 2017. PMID: 27943171 Review.
-
Reliability of Click Chemistry on Drug Discovery: A Personal Account.Chem Rec. 2020 Apr;20(4):253-272. doi: 10.1002/tcr.201900027. Epub 2019 Aug 16. Chem Rec. 2020. PMID: 31419056 Review.
Cited by
-
11H-Benzo[4,5]imidazo[1,2-a]indol-11-one as a New Precursor of Azomethine Ylides: 1,3-Dipolar Cycloaddition Reactions with Cyclopropenes and Maleimides.Int J Mol Sci. 2022 Oct 30;23(21):13202. doi: 10.3390/ijms232113202. Int J Mol Sci. 2022. PMID: 36361988 Free PMC article.
-
Biological Evaluation of 3-Azaspiro[Bicyclo[3.1.0]Hexane-2,5'-Pyrimidines] as Potential Antitumor Agents.Int J Mol Sci. 2022 Sep 15;23(18):10759. doi: 10.3390/ijms231810759. Int J Mol Sci. 2022. PMID: 36142688 Free PMC article.
-
Identification of Spiro-Fused Pyrrolo[3,4-a]pyrrolizines and Tryptanthrines as Potential Antitumor Agents: Synthesis and In Vitro Evaluation.Int J Mol Sci. 2021 Nov 5;22(21):11997. doi: 10.3390/ijms222111997. Int J Mol Sci. 2021. PMID: 34769424 Free PMC article.
References
-
- Akgun H., Us Yilmaz D., Cetin Atalay R., Gozen D. A Series of 2,4(1H,3H)-Quinazolinedione Derivatives: Synthesis and Biological Evaluation as Potential Anticancer Agents. Lett. Drug Des. Discov. 2016;13:64–76. doi: 10.2174/1570180812666150529204909. - DOI
-
- Blass B.E. Basic Principles of Drug Discovery and Development. 1st ed. Academic Press; Boston, MA, USA: 2015. Chapter 1—Drug Discovery and Development: An Overview of Modern Methods and Principles; pp. 1–34.
-
- Marti C., Carreira E.M. Construction of Spiro[pyrrolidine-3,3′-oxindoles]—Recent Applications to the Synthesis of Oxindole Alkaloids. Eur. J. Org. Chem. 2003;12:2209–2219. doi: 10.1002/ejoc.200300050. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

