Differential Proteomic Analysis of Astrocytes and Astrocytes-Derived Extracellular Vesicles from Control and Rai Knockout Mice: Insights into the Mechanisms of Neuroprotection
- PMID: 34360699
- PMCID: PMC8348125
- DOI: 10.3390/ijms22157933
Differential Proteomic Analysis of Astrocytes and Astrocytes-Derived Extracellular Vesicles from Control and Rai Knockout Mice: Insights into the Mechanisms of Neuroprotection
Abstract
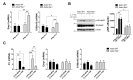
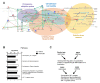
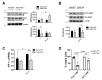
Reactive astrocytes are a hallmark of neurodegenerative disease including multiple sclerosis. It is widely accepted that astrocytes may adopt alternative phenotypes depending on a combination of environmental cues and intrinsic features in a highly plastic and heterogeneous manner. However, we still lack a full understanding of signals and associated signaling pathways driving astrocyte reaction and of the mechanisms by which they drive disease. We have previously shown in the experimental autoimmune encephalomyelitis mouse model that deficiency of the molecular adaptor Rai reduces disease severity and demyelination. Moreover, using primary mouse astrocytes, we showed that Rai contributes to the generation of a pro-inflammatory central nervous system (CNS) microenvironment through the production of nitric oxide and IL-6 and by impairing CD39 activity in response to soluble factors released by encephalitogenic T cells. Here, we investigated the impact of Rai expression on astrocyte function both under basal conditions and in response to IL-17 treatment using a proteomic approach. We found that astrocytes and astrocyte-derived extracellular vesicles contain a set of proteins, to which Rai contributes, that are involved in the regulation of oligodendrocyte differentiation and myelination, nitrogen metabolism, and oxidative stress. The HIF-1α pathway and cellular energetic metabolism were the most statistically relevant molecular pathways and were related to ENOA and HSP70 dysregulation.
Keywords: HIF-1α; IL-17; astrocytes; extracellular vesicles; molecular adaptor; proteomics.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
A T Cell Suppressive Circuitry Mediated by CD39 and Regulated by ShcC/Rai Is Induced in Astrocytes by Encephalitogenic T Cells.Front Immunol. 2019 May 10;10:1041. doi: 10.3389/fimmu.2019.01041. eCollection 2019. Front Immunol. 2019. PMID: 31134091 Free PMC article.
-
The Adaptor Protein Rai/ShcC Promotes Astrocyte-Dependent Inflammation during Experimental Autoimmune Encephalomyelitis.J Immunol. 2016 Jul 15;197(2):480-90. doi: 10.4049/jimmunol.1502063. Epub 2016 Jun 10. J Immunol. 2016. PMID: 27288534
-
Gp130-dependent astrocytic survival is critical for the control of autoimmune central nervous system inflammation.J Immunol. 2011 Jun 1;186(11):6521-31. doi: 10.4049/jimmunol.1001135. Epub 2011 Apr 22. J Immunol. 2011. PMID: 21515788
-
NG2-glia, More Than Progenitor Cells.Adv Exp Med Biol. 2016;949:27-45. doi: 10.1007/978-3-319-40764-7_2. Adv Exp Med Biol. 2016. PMID: 27714683 Review.
-
Astrocyte-Derived Extracellular Vesicles for Ischemic Stroke: Therapeutic Potential and Prospective.Aging Dis. 2024 May 7;15(3):1227-1254. doi: 10.14336/AD.2023.0823-1. Aging Dis. 2024. PMID: 37728588 Free PMC article. Review.
Cited by
-
Digoxin Induces Human Astrocyte Reaction In Vitro.Mol Neurobiol. 2023 Jan;60(1):84-97. doi: 10.1007/s12035-022-03057-1. Epub 2022 Oct 12. Mol Neurobiol. 2023. PMID: 36223047 Free PMC article.
-
Multi-Omics Integrative Approach of Extracellular Vesicles: A Future Challenging Milestone.Proteomes. 2022 Apr 22;10(2):12. doi: 10.3390/proteomes10020012. Proteomes. 2022. PMID: 35645370 Free PMC article. Review.
-
Inflammaging and Brain Aging.Int J Mol Sci. 2024 Sep 30;25(19):10535. doi: 10.3390/ijms251910535. Int J Mol Sci. 2024. PMID: 39408862 Free PMC article. Review.
-
Differential Protein Expression in Extracellular Vesicles Defines Treatment Responders and Non-Responders in Multiple Sclerosis.Int J Mol Sci. 2024 Oct 6;25(19):10761. doi: 10.3390/ijms251910761. Int J Mol Sci. 2024. PMID: 39409091 Free PMC article.
-
Proteomics in Multiple Sclerosis: The Perspective of the Clinician.Int J Mol Sci. 2022 May 5;23(9):5162. doi: 10.3390/ijms23095162. Int J Mol Sci. 2022. PMID: 35563559 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
