Metabolomic Identification of Anticancer Metabolites of Australian Propolis and Proteomic Elucidation of Its Synergistic Mechanisms with Doxorubicin in the MCF7 Cells
- PMID: 34360606
- PMCID: PMC8346082
- DOI: 10.3390/ijms22157840
Metabolomic Identification of Anticancer Metabolites of Australian Propolis and Proteomic Elucidation of Its Synergistic Mechanisms with Doxorubicin in the MCF7 Cells
Abstract
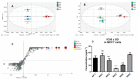
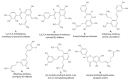
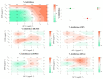
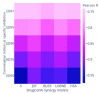
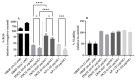
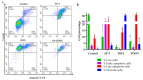
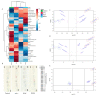
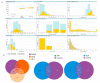
The combination of natural products with standard chemotherapeutic agents offers a promising strategy to enhance the efficacy or reduce the side effects of standard chemotherapy. Doxorubicin (DOX), a standard drug for breast cancer, has several disadvantages, including severe side effects and the development of drug resistance. Recently, we reported the potential bioactive markers of Australian propolis extract (AP-1) and their broad spectrum of pharmacological activities. In the present study, we explored the synergistic interactions between AP-1 and DOX in the MCF7 breast adenocarcinoma cells using different synergy quantitation models. Biochemometric and metabolomics-driven analysis was performed to identify the potential anticancer metabolites in AP-1. The molecular mechanisms of synergy were studied by analysing the apoptotic profile via flow cytometry, apoptotic proteome array and measuring the oxidative status of the MCF7 cells treated with the most synergistic combination. Furthermore, label-free quantification proteomics analysis was performed to decipher the underlying synergistic mechanisms. Five prenylated stilbenes were identified as the key metabolites in the most active AP-1 fraction. Strong synergy was observed when AP-1 was combined with DOX in the ratio of 100:0.29 (w/w) as validated by different synergy quantitation models implemented. AP-1 significantly enhanced the inhibitory effect of DOX against MCF7 cell proliferation in a dose-dependent manner with significant inhibition of the reactive oxygen species (p < 0.0001) compared to DOX alone. AP-1 enabled the reversal of DOX-mediated necrosis to programmed cell death, which may be advantageous to decline DOX-related side effects. AP-1 also significantly enhanced the apoptotic effect of DOX after 24 h of treatment with significant upregulation of catalase, HTRA2/Omi, FADD together with DR5 and DR4 TRAIL-mediated apoptosis (p < 0.05), contributing to the antiproliferative activity of AP-1. Significant upregulation of pro-apoptotic p27, PON2 and catalase with downregulated anti-apoptotic XIAP, HSP60 and HIF-1α, and increased antioxidant proteins (catalase and PON2) may be associated with the improved apoptosis and oxidative status of the synergistic combination-treated MCF7 cells compared to the mono treatments. Shotgun proteomics identified 21 significantly dysregulated proteins in the synergistic combination-treated cells versus the mono treatments. These proteins were involved in the TP53/ATM-regulated non-homologous end-joining pathway and double-strand breaks repairs, recruiting the overexpressed BRCA1 and suppressed RIF1 encoded proteins. The overexpression of UPF2 was noticed in the synergistic combination treatment, which could assist in overcoming doxorubicin resistance-associated long non-coding RNA and metastasis of the MCF7 cells. In conclusion, we identified the significant synergy and highlighted the key molecular pathways in the interaction between AP-1 and DOX in the MCF7 cells together with the AP-1 anticancer metabolites. Further in vivo and clinical studies are warranted on this synergistic combination.
Keywords: MCF7; apoptosis; breast adenocarcinoma; breast cancer; doxorubicin; metabolomics; propolis; proteomics; synergy.
Conflict of interest statement
As a medical research institute, NICM Health Research Institute receives grants and donations from foundations, universities, government agencies, individuals, and industry. Sponsors and donors also provide untied funding to advance the vision and mission of the institute. The authors declare no conflict of interest.
Figures

Similar articles
-
Synergistic Interactions of Cannabidiol with Chemotherapeutic Drugs in MCF7 Cells: Mode of Interaction and Proteomics Analysis of Mechanisms.Int J Mol Sci. 2021 Sep 18;22(18):10103. doi: 10.3390/ijms221810103. Int J Mol Sci. 2021. PMID: 34576262 Free PMC article.
-
Reversing Multidrug Resistance in Chemo-resistant Human Lung Adenocarcinoma (A549/DOX) Cells by Algerian Propolis Through Direct Inhibiting the P-gp Efflux-pump, G0/G1 Cell Cycle Arrest and Apoptosis Induction.Anticancer Agents Med Chem. 2018;18(9):1330-1337. doi: 10.2174/1871520618666180808100800. Anticancer Agents Med Chem. 2018. PMID: 30088453
-
Algerian Propolis Potentiates Doxorubicin Mediated Anticancer Effect Against Human Pancreatic PANC-1 Cancer Cell Line through Cell Cycle Arrest, Apoptosis Induction and P-Glycoprotein Inhibition.Anticancer Agents Med Chem. 2018;18(3):375-387. doi: 10.2174/1871520618666180110143239. Anticancer Agents Med Chem. 2018. PMID: 29318976
-
Effects of propolis and its bioactive components on breast cancer cell pathways and the molecular mechanisms involved.Breast Dis. 2021;40(S1):S15-S25. doi: 10.3233/BD-219003. Breast Dis. 2021. PMID: 34057114 Review.
-
Long non-coding RNAs in the doxorubicin resistance of cancer cells.Cancer Lett. 2021 Jun 28;508:104-114. doi: 10.1016/j.canlet.2021.03.018. Epub 2021 Mar 22. Cancer Lett. 2021. PMID: 33766750 Review.
Cited by
-
Mechanistic Insights into the Anti-Proliferative Action of Gut Microbial Metabolites against Breast Adenocarcinoma Cells.Int J Mol Sci. 2023 Oct 10;24(20):15053. doi: 10.3390/ijms242015053. Int J Mol Sci. 2023. PMID: 37894734 Free PMC article.
-
Research Progress on Therapeutic Effect and Mechanism of Propolis on Wound Healing.Evid Based Complement Alternat Med. 2022 Jul 21;2022:5798941. doi: 10.1155/2022/5798941. eCollection 2022. Evid Based Complement Alternat Med. 2022. PMID: 35911156 Free PMC article. Review.
-
Impact of a Functional Dairy Powder and Its Primary Component on the Growth of Pathogenic and Probiotic Gut Bacteria and Human Coronavirus 229E.Int J Mol Sci. 2024 Aug 29;25(17):9353. doi: 10.3390/ijms25179353. Int J Mol Sci. 2024. PMID: 39273301 Free PMC article.
-
Pyroptosis and chemical classification of pyroptotic agents.Mol Divers. 2024 Sep 24. doi: 10.1007/s11030-024-10987-6. Online ahead of print. Mol Divers. 2024. PMID: 39316325 Review.
-
The Potential Use of Propolis as an Adjunctive Therapy in Breast Cancers.Integr Cancer Ther. 2022 Jan-Dec;21:15347354221096868. doi: 10.1177/15347354221096868. Integr Cancer Ther. 2022. PMID: 35593403 Free PMC article.
References
-
- Seddon B., Strauss S.J., Whelan J., Leahy M., Woll P.J., Cowie F., Rothermundt C., Wood Z., Benson C., Ali N., et al. Gemcitabine and docetaxel versus doxorubicin as first-line treatment in previously untreated advanced unresectable or metastatic soft-tissue sarcomas (GeDDiS): A randomised controlled phase 3 trial. Lancet Oncol. 2017;18:1397–1410. doi: 10.1016/S1470-2045(17)30622-8. - DOI - PMC - PubMed
-
- McMeekin S., Dizon D., Barter J., Scambia G., Manzyuk L., Lisyanskaya A., Oaknin A., Ringuette S., Mukhopadhyay P., Rosenberg J., et al. Phase III randomized trial of second-line ixabepilone versus paclitaxel or doxorubicin in women with advanced endometrial cancer. Gynecol. Oncol. 2015;138:18–23. doi: 10.1016/j.ygyno.2015.04.026. - DOI - PubMed
-
- Tap W.D., Wagner A.J., Schoffski P., Martin-Broto J., Krarup-Hansen A., Ganjoo K.N., Yen C.C., Abdul Razak A.R., Spira A., Kawai A., et al. Effect of Doxorubicin Plus Olaratumab vs. Doxorubicin Plus Placebo on Survival in Patients With Advanced Soft Tissue Sarcomas: The ANNOUNCE Randomized Clinical Trial. JAMA. 2020;323:1266–1276. doi: 10.1001/jama.2020.1707. - DOI - PMC - PubMed
-
- Blum J.L., Flynn P.J., Yothers G., Asmar L., Geyer C.E., Jr., Jacobs S.A., Robert N.J., Hopkins J.O., O’Shaughnessy J.A., Dang C.T., et al. Anthracyclines in Early Breast Cancer: The ABC Trials-USOR 06-090, NSABP B-46-I/USOR 07132, and NSABP B-49 (NRG Oncology) J. Clin. Oncol. 2017;35:2647–2655. doi: 10.1200/JCO.2016.71.4147. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

