Involvement of HIF-1α in the Detection, Signaling, and Repair of DNA Double-Strand Breaks after Photon and Carbon-Ion Irradiation
- PMID: 34359734
- PMCID: PMC8345054
- DOI: 10.3390/cancers13153833
Involvement of HIF-1α in the Detection, Signaling, and Repair of DNA Double-Strand Breaks after Photon and Carbon-Ion Irradiation
Abstract
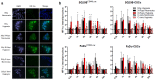
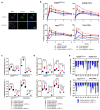
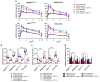
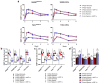
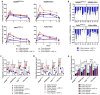
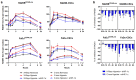
Hypoxia-Inducible Factor 1α (HIF-1α), which promotes cancer cell survival, is the main regulator of oxygen homeostasis. Hypoxia combined with photon and carbon ion irradiation (C-ions) stabilizes HIF-1α. Silencing HIF-1α under hypoxia leads to substantial radiosensitization of Head-and-Neck Squamous Cell Carcinoma (HNSCC) cells after both photons and C-ions. Thus, this study aimed to clarify a potential involvement of HIF-1α in the detection, signaling, and repair of DNA Double-Strand-Breaks (DSBs) in response to both irradiations, in two HNSCC cell lines and their subpopulations of Cancer-Stem Cells (CSCs). After confirming the nucleoshuttling of HIF-1α in response to both exposure under hypoxia, we showed that silencing HIF-1α in non-CSCs and CSCs decreased the initiation of the DSB detection (P-ATM), and increased the residual phosphorylated H2AX (γH2AX) foci. While HIF-1α silencing did not modulate 53BP1 expression, P-DNA-PKcs (NHEJ-c) and RAD51 (HR) signals decreased. Altogether, our experiments demonstrate the involvement of HIF-1α in the detection and signaling of DSBs, but also in the main repair pathways (NHEJ-c and HR), without favoring one of them. Combining HIF-1α silencing with both types of radiation could therefore present a potential therapeutic benefit of targeting CSCs mostly present in tumor hypoxic niches.
Keywords: DNA repair; cancer stem cells; carbon ions; double-strand breaks; homologous recombination; hypoxia; hypoxia-inducible factor 1; irradiations; non-homologous end joining pathway; photons.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Impact of hypoxia on the double-strand break repair after photon and carbon ion irradiation of radioresistant HNSCC cells.Sci Rep. 2020 Dec 7;10(1):21357. doi: 10.1038/s41598-020-78354-7. Sci Rep. 2020. PMID: 33288855 Free PMC article.
-
Differential pattern of HIF-1α expression in HNSCC cancer stem cells after carbon ion or photon irradiation: one molecular explanation of the oxygen effect.Br J Cancer. 2017 May 9;116(10):1340-1349. doi: 10.1038/bjc.2017.100. Epub 2017 Apr 13. Br J Cancer. 2017. PMID: 28407653 Free PMC article.
-
The major DNA repair pathway after both proton and carbon-ion radiation is NHEJ, but the HR pathway is more relevant in carbon ions.Radiat Res. 2015 Mar;183(3):345-56. doi: 10.1667/RR13904.1. Epub 2015 Mar 4. Radiat Res. 2015. PMID: 25738894 Free PMC article.
-
Roles for 53BP1 in the repair of radiation-induced DNA double strand breaks.DNA Repair (Amst). 2020 Sep;93:102915. doi: 10.1016/j.dnarep.2020.102915. DNA Repair (Amst). 2020. PMID: 33087281 Review.
-
Roles for the DNA-PK complex and 53BP1 in protecting ends from resection during DNA double-strand break repair.J Radiat Res. 2020 Sep 8;61(5):718-726. doi: 10.1093/jrr/rraa053. J Radiat Res. 2020. PMID: 32779701 Free PMC article. Review.
Cited by
-
Tumor cell plasticity in targeted therapy-induced resistance: mechanisms and new strategies.Signal Transduct Target Ther. 2023 Mar 11;8(1):113. doi: 10.1038/s41392-023-01383-x. Signal Transduct Target Ther. 2023. PMID: 36906600 Free PMC article. Review.
-
Role of Mitochondria in Radiation Responses: Epigenetic, Metabolic, and Signaling Impacts.Int J Mol Sci. 2021 Oct 13;22(20):11047. doi: 10.3390/ijms222011047. Int J Mol Sci. 2021. PMID: 34681703 Free PMC article. Review.
-
Prognostic Value of Serum Transferrin Level before Radiotherapy on Radio-Sensitivity and Survival in Patients with Nasopharyngeal Carcinoma.J Pers Med. 2023 Mar 13;13(3):511. doi: 10.3390/jpm13030511. J Pers Med. 2023. PMID: 36983693 Free PMC article.
-
Carbon Ions for Hypoxic Tumors: Are We Making the Most of Them?Cancers (Basel). 2023 Sep 9;15(18):4494. doi: 10.3390/cancers15184494. Cancers (Basel). 2023. PMID: 37760464 Free PMC article. Review.
-
Depletion of HIF-1α by Inducible Cre/loxP Increases the Sensitivity of Cultured Murine Hepatocytes to Ionizing Radiation in Hypoxia.Cells. 2022 May 18;11(10):1671. doi: 10.3390/cells11101671. Cells. 2022. PMID: 35626708 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

