Environmental enteric dysfunction induces regulatory T cells that inhibit local CD4+ T cell responses and impair oral vaccine efficacy
- PMID: 34348118
- PMCID: PMC8415388
- DOI: 10.1016/j.immuni.2021.07.005
Environmental enteric dysfunction induces regulatory T cells that inhibit local CD4+ T cell responses and impair oral vaccine efficacy
Abstract
Environmental enteric dysfunction (EED) is a gastrointestinal inflammatory disease caused by malnutrition and chronic infection. EED is associated with stunting in children and reduced efficacy of oral vaccines. To study the mechanisms of oral vaccine failure during EED, we developed a microbiota- and diet-dependent mouse EED model. Analysis of E. coli-labile toxin vaccine-specific CD4+ T cells in these mice revealed impaired CD4+ T cell responses in the small intestine and but not the lymph nodes. EED mice exhibited increased frequencies of small intestine-resident RORγT+FOXP3+ regulatory T (Treg) cells. Targeted deletion of RORγT from Treg cells restored small intestinal vaccine-specific CD4 T cell responses and vaccine-mediated protection upon challenge. However, ablation of RORγT+FOXP3+ Treg cells made mice more susceptible to EED-induced stunting. Our findings provide insight into the poor efficacy of oral vaccines in EED and highlight how RORγT+FOXP3+ Treg cells can regulate intestinal immunity while leaving systemic responses intact.
Keywords: AIEC; E coli; EED; RORgt Treg; Tregs; diet; intestinal; microbiota; oral vaccination; stunting.
Copyright © 2021 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures


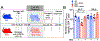


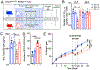
Similar articles
-
RORγt Promotes Foxp3 Expression by Antagonizing the Effector Program in Colonic Regulatory T Cells.J Immunol. 2021 Oct 15;207(8):2027-2038. doi: 10.4049/jimmunol.2100175. Epub 2021 Sep 13. J Immunol. 2021. PMID: 34518282 Free PMC article.
-
A GMCSF-Neuroantigen Tolerogenic Vaccine Elicits Systemic Lymphocytosis of CD4+ CD25high FOXP3+ Regulatory T Cells in Myelin-Specific TCR Transgenic Mice Contingent Upon Low-Efficiency T Cell Antigen Receptor Recognition.Front Immunol. 2019 Jan 10;9:3119. doi: 10.3389/fimmu.2018.03119. eCollection 2018. Front Immunol. 2019. PMID: 30687323 Free PMC article.
-
Listeriolysin O expressed in a bacterial vaccine suppresses CD4+CD25high regulatory T cell function in vivo.J Immunol. 2007 Aug 1;179(3):1532-41. doi: 10.4049/jimmunol.179.3.1532. J Immunol. 2007. PMID: 17641019
-
The Functional Stability of FOXP3 and RORγt in Treg and Th17 and Their Therapeutic Applications.Adv Protein Chem Struct Biol. 2017;107:155-189. doi: 10.1016/bs.apcsb.2016.10.002. Epub 2016 Dec 15. Adv Protein Chem Struct Biol. 2017. PMID: 28215223 Review.
-
Exploring the relationship between environmental enteric dysfunction and oral vaccine responses.Future Microbiol. 2018 Jul;13(9):1055-1070. doi: 10.2217/fmb-2018-0016. Epub 2018 Jun 21. Future Microbiol. 2018. PMID: 29926747 Free PMC article. Review.
Cited by
-
Update on Early-Life T Cells: Impact on Oral Rotavirus Vaccines.Viruses. 2024 May 22;16(6):818. doi: 10.3390/v16060818. Viruses. 2024. PMID: 38932111 Free PMC article. Review.
-
Bile acids impact the microbiota, host, and C. difficile dynamics providing insight into mechanisms of efficacy of FMTs and microbiota-focused therapeutics.Gut Microbes. 2024 Jan-Dec;16(1):2393766. doi: 10.1080/19490976.2024.2393766. Epub 2024 Sep 3. Gut Microbes. 2024. PMID: 39224076 Free PMC article. Review.
-
Plasma Kynurenine to Tryptophan Ratio Is Not Associated with Undernutrition in Adults but Reduced after Nutrition Intervention: Results from a Community-Based Study in Bangladesh.Nutrients. 2022 Apr 20;14(9):1708. doi: 10.3390/nu14091708. Nutrients. 2022. PMID: 35565678 Free PMC article.
-
Impact of childhood malnutrition and intestinal microbiota on MDR infections.JAC Antimicrob Resist. 2023 Apr 24;5(2):dlad051. doi: 10.1093/jacamr/dlad051. eCollection 2023 Apr. JAC Antimicrob Resist. 2023. PMID: 37102119 Free PMC article. Review.
-
LAMTOR1 decreased exosomal PD-L1 to enhance immunotherapy efficacy in non-small cell lung cancer.Mol Cancer. 2024 Sep 2;23(1):184. doi: 10.1186/s12943-024-02099-4. Mol Cancer. 2024. PMID: 39223601 Free PMC article.
References
-
- Barnich N, and Darfeuille-Michaud A (2007). Adherent-invasive Escherichia coli and Crohn's disease. Curr Opin Gastroenterol 23, 16–20. - PubMed
-
- Bhattacharjee A, and Hand TW (2018). Role of nutrition, infection, and the microbiota in the efficacy of oral vaccines. Clin Sci (Lond) 132, 1169–1177. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

