Impact of early corticosteroids on 60-day mortality in critically ill patients with COVID-19: A multicenter cohort study of the OUTCOMEREA network
- PMID: 34347836
- PMCID: PMC8336847
- DOI: 10.1371/journal.pone.0255644
Impact of early corticosteroids on 60-day mortality in critically ill patients with COVID-19: A multicenter cohort study of the OUTCOMEREA network
Abstract
Objectives: In severe COVID-19 pneumonia, the appropriate timing and dosing of corticosteroids (CS) is not known. Patient subgroups for which CS could be more beneficial also need appraisal. The aim of this study was to assess the effect of early CS in COVID-19 pneumonia patients admitted to the ICU on the occurrence of 60-day mortality, ICU-acquired-bloodstream infections(ICU-BSI), and hospital-acquired pneumonia and ventilator-associated pneumonia(HAP-VAP).
Methods: We included patients with COVID-19 pneumonia admitted to 11 ICUs belonging to the French OutcomeReaTM network from January to May 2020. We used survival models with ponderation with inverse probability of treatment weighting (IPTW).
Results: The study population comprised 303 patients having a median age of 61.6 (53-70) years of whom 78.8% were male and 58.6% had at least one comorbidity. The median SAPS II was 33 (25-44). Invasive mechanical ventilation was required in 34.8% of the patients. Sixty-six (21.8%) patients were in the Early-C subgroup. Overall, 60-day mortality was 29.4%. The risks of 60-day mortality (IPTWHR = 0.86;95% CI 0.54 to 1.35, p = 0.51), ICU-BSI and HAP-VAP were similar in the two groups. Importantly, early CS treatment was associated with a lower mortality rate in patients aged 60 years or more (IPTWHR, 0.53;95% CI, 0.3-0.93; p = 0.03). In contrast, CS was associated with an increased risk of death in patients younger than 60 years without inflammation on admission (IPTWHR = 5.01;95% CI, 1.05, 23.88; p = 0.04).
Conclusion: For patients with COVID-19 pneumonia, early CS treatment was not associated with patient survival. Interestingly, inflammation and age can significantly influence the effect of CS.
Conflict of interest statement
The authors have read the journal’s policy and have the following competing interests: SR is a paid employee of ICUREsearch. There are no patents, products in development or marketed products associated with this research to declare. This does not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures
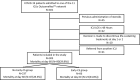

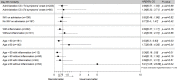
Similar articles
-
Association Between Early Treatment With Tocilizumab and Mortality Among Critically Ill Patients With COVID-19.JAMA Intern Med. 2021 Jan 1;181(1):41-51. doi: 10.1001/jamainternmed.2020.6252. JAMA Intern Med. 2021. PMID: 33080002 Free PMC article.
-
Early corticosteroids are associated with lower mortality in critically ill patients with COVID-19: a cohort study.Crit Care. 2021 Jan 4;25(1):2. doi: 10.1186/s13054-020-03422-3. Crit Care. 2021. PMID: 33397463 Free PMC article.
-
Risk Factors Associated With Mortality Among Patients With COVID-19 in Intensive Care Units in Lombardy, Italy.JAMA Intern Med. 2020 Oct 1;180(10):1345-1355. doi: 10.1001/jamainternmed.2020.3539. JAMA Intern Med. 2020. PMID: 32667669 Free PMC article.
-
Oral hygiene care for critically ill patients to prevent ventilator-associated pneumonia.Cochrane Database Syst Rev. 2013 Aug 13;(8):CD008367. doi: 10.1002/14651858.CD008367.pub2. Cochrane Database Syst Rev. 2013. Update in: Cochrane Database Syst Rev. 2016 Oct 25;10:CD008367. doi: 10.1002/14651858.CD008367.pub3 PMID: 23939759 Updated. Review.
-
Interventions for preventing critical illness polyneuropathy and critical illness myopathy.Cochrane Database Syst Rev. 2014 Jan 30;2014(1):CD006832. doi: 10.1002/14651858.CD006832.pub3. Cochrane Database Syst Rev. 2014. PMID: 24477672 Free PMC article. Review.
Cited by
-
Co-infection and ICU-acquired infection in COIVD-19 ICU patients: a secondary analysis of the UNITE-COVID data set.Crit Care. 2022 Aug 3;26(1):236. doi: 10.1186/s13054-022-04108-8. Crit Care. 2022. PMID: 35922860 Free PMC article.
-
Mortality, incidence, and microbiological documentation of ventilated acquired pneumonia (VAP) in critically ill patients with COVID-19 or influenza.Ann Intensive Care. 2023 Oct 30;13(1):108. doi: 10.1186/s13613-023-01207-9. Ann Intensive Care. 2023. PMID: 37902869 Free PMC article.
-
Corticosteroid Therapy in COVID-19 Associated With In-hospital Mortality in Geriatric Patients: A Propensity Matched Cohort Study.J Gerontol A Biol Sci Med Sci. 2022 Jul 5;77(7):1352-1360. doi: 10.1093/gerona/glac084. J Gerontol A Biol Sci Med Sci. 2022. PMID: 35395678 Free PMC article.
-
Major candidate variables to guide personalised treatment with steroids in critically ill patients with COVID-19: CIBERESUCICOVID study.Intensive Care Med. 2022 Jul;48(7):850-864. doi: 10.1007/s00134-022-06726-w. Epub 2022 Jun 21. Intensive Care Med. 2022. PMID: 35727348 Free PMC article.
-
Antibiotic Usage in the COVID-19 Intensive Care Unit of an Infectious Diseases Hospital from Nord-Eastern Romania.Medicina (Kaunas). 2023 Mar 24;59(4):645. doi: 10.3390/medicina59040645. Medicina (Kaunas). 2023. PMID: 37109601 Free PMC article.
References
-
- Xie J, Wu W, Li S, Hu Y, Hu M, Li J, et al.. Clinical characteristics and outcomes of critically ill patients with novel coronavirus infectious disease (COVID-19) in China: a retrospective multicenter study. Intensive Care Med. 2020. [cited 28 Aug 2020]. doi: 10.1007/s00134-020-06211-2 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

