Engineering of the current nucleoside-modified mRNA-LNP vaccines against SARS-CoV-2
- PMID: 34343897
- PMCID: PMC8299225
- DOI: 10.1016/j.biopha.2021.111953
Engineering of the current nucleoside-modified mRNA-LNP vaccines against SARS-CoV-2
Abstract
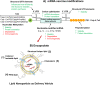
Currently, there are over 230 different COVID-19 vaccines under development around the world. At least three decades of scientific development in RNA biology, immunology, structural biology, genetic engineering, chemical modification, and nanoparticle technologies allowed the accelerated development of fully synthetic messenger RNA (mRNA)-based vaccines within less than a year since the first report of a SARS-CoV-2 infection. mRNA-based vaccines have been shown to elicit broadly protective immune responses, with the added advantage of being amenable to rapid and flexible manufacturing processes. This review recapitulates current advances in engineering the first two SARS-CoV-2-spike-encoding nucleoside-modified mRNA vaccines, highlighting the strategies followed to potentiate their effectiveness and safety, thus facilitating an agile response to the current COVID-19 pandemic.
Keywords: LNP, vaccines; MRNA; Nucleoside-modified; SARS-CoV-2; Spike protein.
Copyright © 2021 The Authors. Published by Elsevier Masson SAS.. All rights reserved.
Conflict of interest statement
None
Figures
Similar articles
-
Messenger RNA vaccines against SARS-CoV-2.Cell. 2021 Mar 18;184(6):1401. doi: 10.1016/j.cell.2020.12.039. Epub 2021 Jan 13. Cell. 2021. PMID: 33740443 Free PMC article.
-
SARS-CoV-2 mRNA Vaccines Elicit Different Responses in Immunologically Naïve and Pre-Immune Humans.Front Immunol. 2021 Sep 27;12:728021. doi: 10.3389/fimmu.2021.728021. eCollection 2021. Front Immunol. 2021. PMID: 34646267 Free PMC article.
-
Production and Evaluation of Nucleoside-Modified mRNA Vaccines for Infectious Diseases.Methods Mol Biol. 2024;2786:167-181. doi: 10.1007/978-1-0716-3770-8_7. Methods Mol Biol. 2024. PMID: 38814394
-
The Nanoparticle-Enabled Success of COVID-19 mRNA Vaccines and the Promise of Microneedle Platforms for Pandemic Vaccine Response.DNA Cell Biol. 2022 Jan;41(1):25-29. doi: 10.1089/dna.2021.0538. Epub 2021 Dec 24. DNA Cell Biol. 2022. PMID: 34958232 Free PMC article. Review.
-
Coronavirus Disease (COVID-19): Current Status and Prospects for Drug and Vaccine Development.Arch Med Res. 2021 Jan;52(1):15-24. doi: 10.1016/j.arcmed.2020.09.010. Epub 2020 Sep 10. Arch Med Res. 2021. PMID: 32950264 Free PMC article. Review.
Cited by
-
Two Years into the COVID-19 Pandemic: Lessons Learned.ACS Infect Dis. 2022 Sep 9;8(9):1758-1814. doi: 10.1021/acsinfecdis.2c00204. Epub 2022 Aug 8. ACS Infect Dis. 2022. PMID: 35940589 Free PMC article. Review.
-
Principles for designing an optimal mRNA lipid nanoparticle vaccine.Curr Opin Biotechnol. 2022 Feb;73:329-336. doi: 10.1016/j.copbio.2021.09.016. Epub 2021 Oct 26. Curr Opin Biotechnol. 2022. PMID: 34715546 Free PMC article. Review.
-
Lipid nanoparticles in the development of mRNA vaccines for COVID-19.J Drug Deliv Sci Technol. 2022 Aug;74:103553. doi: 10.1016/j.jddst.2022.103553. Epub 2022 Jun 28. J Drug Deliv Sci Technol. 2022. PMID: 35783677 Free PMC article. Review.
-
Challenges and Scientific Prospects of the Newest Generation of mRNA-Based Vaccines against SARS-CoV-2.Life (Basel). 2021 Aug 31;11(9):907. doi: 10.3390/life11090907. Life (Basel). 2021. PMID: 34575056 Free PMC article. Review.
-
Nucleic Acids as Biotools at the Interface between Chemistry and Nanomedicine in the COVID-19 Era.Int J Mol Sci. 2022 Apr 14;23(8):4359. doi: 10.3390/ijms23084359. Int J Mol Sci. 2022. PMID: 35457177 Free PMC article. Review.
References
-
- Wolff J.A., Malone R.W., Williams P., Chong W., Acsadi G., Jani A., Felgner P.L. Direct gene transfer into mouse muscle in vivo. Science. 1990;247(4949 Pt 1):1465–1468. - PubMed
-
- Jackson L.A., Anderson E.J., Rouphael N.G., Roberts P.C., Makhene M., Coler R.N., McCullough M.P., Chappell J.D., Denison M.R., Stevens L.J., Pruijssers A.J., McDermott A., Flach B., Doria-Rose N.A., Corbett K.S., Morabito K.M., O’Dell S., Schmidt S.D., Swanson P.A., 2nd, Padilla M., Mascola J.R., Neuzil K.M., Bennett H., Sun W., Peters E., Makowski M., Albert J., Cross K., Buchanan W., Pikaart-Tautges R., Ledgerwood J.E., Graham B.S., Beigel J.H. An mRNA vaccine against SARS-CoV-2 - preliminary report. N. Engl. J. Med. 2020;383(20):1920–1931. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

