Viruses, SUMO, and immunity: the interplay between viruses and the host SUMOylation system
- PMID: 34342851
- PMCID: PMC8330205
- DOI: 10.1007/s13365-021-00995-9
Viruses, SUMO, and immunity: the interplay between viruses and the host SUMOylation system
Abstract
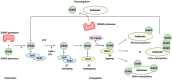
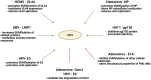
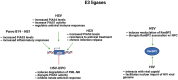
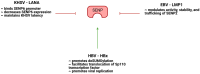
The conjugation of small ubiquitin-like modifier (SUMO) proteins to substrates is a well-described post-translational modification that regulates protein activity, subcellular localization, and protein-protein interactions for a variety of downstream cellular activities. Several studies describe SUMOylation as an essential post-translational modification for successful viral infection across a broad range of viruses, including RNA and DNA viruses, both enveloped and un-enveloped. These viruses include but are not limited to herpes viruses, human immunodeficiency virus-1, and coronaviruses. In addition to the SUMOylation of viral proteins during infection, evidence shows that viruses manipulate the SUMO pathway for host protein SUMOylation. SUMOylation of host and viral proteins greatly impacts host innate immunity through viral manipulation of the host SUMOylation machinery to promote viral replication and pathogenesis. Other post-translational modifications like phosphorylation can also modulate SUMO function. For example, phosphorylation of COUP-TF interacting protein 2 (CTIP2) leads to its SUMOylation and subsequent proteasomal degradation. The SUMOylation of CTIP2 and subsequent degradation prevents CTIP2-mediated recruitment of a multi-enzymatic complex to the HIV-1 promoter that usually prevents the transcription of integrated viral DNA. Thus, the "SUMO switch" could have implications for CTIP2-mediated transcriptional repression of HIV-1 in latency and viral persistence. In this review, we describe the consequences of SUMO in innate immunity and then focus on the various ways that viral pathogens have evolved to hijack the conserved SUMO machinery. Increased understanding of the many roles of SUMOylation in viral infections can lead to novel insight into the regulation of viral pathogenesis with the potential to uncover new targets for antiviral therapies.
Keywords: Infection; Post-translational modifications; SUMOylation; Virus.
© 2021. Journal of NeuroVirology, Inc.
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Interplay between viruses and host sumoylation pathways.Nat Rev Microbiol. 2013 Jun;11(6):400-11. doi: 10.1038/nrmicro3015. Epub 2013 Apr 29. Nat Rev Microbiol. 2013. PMID: 23624814 Review.
-
SUMO Ligase Protein Inhibitor of Activated STAT1 (PIAS1) Is a Constituent Promyelocytic Leukemia Nuclear Body Protein That Contributes to the Intrinsic Antiviral Immune Response to Herpes Simplex Virus 1.J Virol. 2016 Jun 10;90(13):5939-5952. doi: 10.1128/JVI.00426-16. Print 2016 Jul 1. J Virol. 2016. PMID: 27099310 Free PMC article.
-
Viral Interplay with the Host Sumoylation System.Adv Exp Med Biol. 2017;963:359-388. doi: 10.1007/978-3-319-50044-7_21. Adv Exp Med Biol. 2017. PMID: 28197923 Free PMC article. Review.
-
Novel Role for Protein Inhibitor of Activated STAT 4 (PIAS4) in the Restriction of Herpes Simplex Virus 1 by the Cellular Intrinsic Antiviral Immune Response.J Virol. 2016 Apr 14;90(9):4807-4826. doi: 10.1128/JVI.03055-15. Print 2016 May. J Virol. 2016. PMID: 26937035 Free PMC article.
-
Viral DNA Binding Protein SUMOylation Promotes PML Nuclear Body Localization Next to Viral Replication Centers.mBio. 2020 Mar 17;11(2):e00049-20. doi: 10.1128/mBio.00049-20. mBio. 2020. PMID: 32184235 Free PMC article.
Cited by
-
Identification of SARS-CoV-2 Main Protease (Mpro) Cleavage Sites Using Two-Dimensional Electrophoresis and In Silico Cleavage Site Prediction.Int J Mol Sci. 2023 Feb 6;24(4):3236. doi: 10.3390/ijms24043236. Int J Mol Sci. 2023. PMID: 36834648 Free PMC article.
-
Interplay between Lipid Metabolism, Lipid Droplets, and DNA Virus Infections.Cells. 2022 Jul 17;11(14):2224. doi: 10.3390/cells11142224. Cells. 2022. PMID: 35883666 Free PMC article. Review.
-
RanBP2/Nup358 Mediates Sumoylation of STAT1 and Antagonizes Interferon-α-Mediated Antiviral Innate Immunity.Int J Mol Sci. 2023 Dec 25;25(1):299. doi: 10.3390/ijms25010299. Int J Mol Sci. 2023. PMID: 38203469 Free PMC article.
-
Potential role of PIM1 inhibition in the treatment of SARS-CoV-2 infection.J Genet Eng Biotechnol. 2023 May 22;21(1):65. doi: 10.1186/s43141-023-00520-x. J Genet Eng Biotechnol. 2023. PMID: 37211584 Free PMC article.
-
SARS-CoV-2 Nucleocapsid Protein Induces Tau Pathological Changes That Can Be Counteracted by SUMO2.Int J Mol Sci. 2024 Jun 28;25(13):7169. doi: 10.3390/ijms25137169. Int J Mol Sci. 2024. PMID: 39000276 Free PMC article.
References
-
- Ait-Ammar A, Bellefroid M, Daouad F, Martinelli V, Van Assche J, Wallet C, Rodari A, De Rovere M, Fahrenkrog B, Schwartz C, Van Lint C, Gautier V, Rohr O. Inhibition of HIV-1 gene transcription by KAP1 in myeloid lineage. Sci Rep. 2021;11(1):2692. doi: 10.1038/s41598-021-82164-w. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

