Mechanisms of temozolomide resistance in glioblastoma - a comprehensive review
- PMID: 34337348
- PMCID: PMC8319838
- DOI: 10.20517/cdr.2020.79
Mechanisms of temozolomide resistance in glioblastoma - a comprehensive review
Abstract
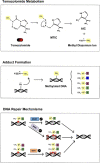
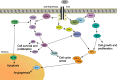
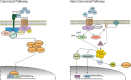
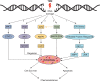
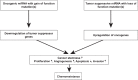
Glioblastoma (GBM) is the most common primary malignant brain tumor in adults and has an exceedingly low median overall survival of only 15 months. Current standard-of-care for GBM consists of gross total surgical resection followed by radiation with concurrent and adjuvant chemotherapy. Temozolomide (TMZ) is the first-choice chemotherapeutic agent in GBM; however, the development of resistance to TMZ often becomes the limiting factor in effective treatment. While O6-methylguanine-DNA methyltransferase repair activity and uniquely resistant populations of glioma stem cells are the most well-known contributors to TMZ resistance, many other molecular mechanisms have come to light in recent years. Key emerging mechanisms include the involvement of other DNA repair systems, aberrant signaling pathways, autophagy, epigenetic modifications, microRNAs, and extracellular vesicle production. This review aims to provide a comprehensive overview of the clinically relevant molecular mechanisms and their extensive interconnections to better inform efforts to combat TMZ resistance.
Keywords: Glioblastoma; chemoresistance; molecular mechanisms; temozolomide.
Conflict of interest statement
Conflicts of interest All authors declared that there are no conflicts of interest.
Figures

Similar articles
-
The histone demethylase KDM5A is a key factor for the resistance to temozolomide in glioblastoma.Cell Cycle. 2015;14(21):3418-29. doi: 10.1080/15384101.2015.1090063. Cell Cycle. 2015. PMID: 26566863 Free PMC article.
-
20(S)-ginsenoside-Rg3 reverses temozolomide resistance and restrains epithelial-mesenchymal transition progression in glioblastoma.Cancer Sci. 2019 Jan;110(1):389-400. doi: 10.1111/cas.13881. Epub 2018 Dec 14. Cancer Sci. 2019. PMID: 30431207 Free PMC article.
-
Pharmacological inhibition of poly(ADP-ribose) polymerase-1 modulates resistance of human glioblastoma stem cells to temozolomide.BMC Cancer. 2014 Mar 5;14:151. doi: 10.1186/1471-2407-14-151. BMC Cancer. 2014. PMID: 24593254 Free PMC article.
-
Overcoming Resistance to Temozolomide in Glioblastoma: A Scoping Review of Preclinical and Clinical Data.Life (Basel). 2024 May 24;14(6):673. doi: 10.3390/life14060673. Life (Basel). 2024. PMID: 38929657 Free PMC article. Review.
-
Elucidating the mechanisms of Temozolomide resistance in gliomas and the strategies to overcome the resistance.Biochim Biophys Acta Rev Cancer. 2021 Dec;1876(2):188616. doi: 10.1016/j.bbcan.2021.188616. Epub 2021 Aug 20. Biochim Biophys Acta Rev Cancer. 2021. PMID: 34419533 Review.
Cited by
-
Evaluation of Microvascular Density in Glioblastomas in Relation to p53 and Ki67 Immunoexpression.Int J Mol Sci. 2024 Jun 20;25(12):6810. doi: 10.3390/ijms25126810. Int J Mol Sci. 2024. PMID: 38928515 Free PMC article.
-
Targeting RTK-PI3K-mTOR Axis in Gliomas: An Update.Int J Mol Sci. 2021 May 5;22(9):4899. doi: 10.3390/ijms22094899. Int J Mol Sci. 2021. PMID: 34063168 Free PMC article. Review.
-
Metabolic Reprogramming in Glioblastoma Multiforme: A Review of Pathways and Therapeutic Targets.Cells. 2024 Sep 19;13(18):1574. doi: 10.3390/cells13181574. Cells. 2024. PMID: 39329757 Free PMC article. Review.
-
NEO212, temozolomide conjugated to NEO100, exerts superior therapeutic activity over temozolomide in preclinical chemoradiation models of glioblastoma.Neurooncol Adv. 2024 Jun 11;6(1):vdae095. doi: 10.1093/noajnl/vdae095. eCollection 2024 Jan-Dec. Neurooncol Adv. 2024. PMID: 39022643 Free PMC article.
-
Triggering of endoplasmic reticulum stress via ATF4-SPHK1 signaling promotes glioblastoma invasion and chemoresistance.Cell Death Dis. 2024 Aug 1;15(8):552. doi: 10.1038/s41419-024-06936-8. Cell Death Dis. 2024. PMID: 39090107 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
