Advanced human-relevant in vitro pulmonary platforms for respiratory therapeutics
- PMID: 34331989
- PMCID: PMC7611797
- DOI: 10.1016/j.addr.2021.113901
Advanced human-relevant in vitro pulmonary platforms for respiratory therapeutics
Abstract
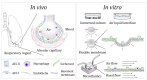
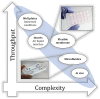
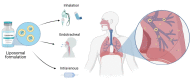
Over the past years, advanced in vitro pulmonary platforms have witnessed exciting developments that are pushing beyond traditional preclinical cell culture methods. Here, we discuss ongoing efforts in bridging the gap between in vivo and in vitro interfaces and identify some of the bioengineering challenges that lie ahead in delivering new generations of human-relevant in vitro pulmonary platforms. Notably, in vitro strategies using foremost lung-on-chips and biocompatible "soft" membranes have focused on platforms that emphasize phenotypical endpoints recapitulating key physiological and cellular functions. We review some of the most recent in vitro studies underlining seminal therapeutic screens and translational applications and open our discussion to promising avenues of pulmonary therapeutic exploration focusing on liposomes. Undeniably, there still remains a recognized trade-off between the physiological and biological complexity of these in vitro lung models and their ability to deliver assays with throughput capabilities. The upcoming years are thus anticipated to see further developments in broadening the applicability of such in vitro systems and accelerating therapeutic exploration for drug discovery and translational medicine in treating respiratory disorders.
Keywords: Aerosols; In vitro; Inhalation therapy; Lung diseases; Lung-on-chips; Preclinical models.
Copyright © 2021 Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

Similar articles
-
Advanced in vitro lung-on-chip platforms for inhalation assays: From prospect to pipeline.Eur J Pharm Biopharm. 2019 Nov;144:11-17. doi: 10.1016/j.ejpb.2019.09.006. Epub 2019 Sep 6. Eur J Pharm Biopharm. 2019. PMID: 31499161 Free PMC article. Review.
-
Advanced lung organoids for respiratory system and pulmonary disease modeling.J Tissue Eng. 2024 Feb 22;15:20417314241232502. doi: 10.1177/20417314241232502. eCollection 2024 Jan-Dec. J Tissue Eng. 2024. PMID: 38406820 Free PMC article. Review.
-
Human Multi-Compartment Airways-on-Chip Platform for Emulating Respiratory Airborne Transmission: From Nose to Pulmonary Acini.Front Physiol. 2022 Mar 8;13:853317. doi: 10.3389/fphys.2022.853317. eCollection 2022. Front Physiol. 2022. PMID: 35350687 Free PMC article.
-
Modifying and Integrating in vitro and ex vivo Respiratory Models for Inhalation Drug Screening.Front Bioeng Biotechnol. 2020 Oct 23;8:581995. doi: 10.3389/fbioe.2020.581995. eCollection 2020. Front Bioeng Biotechnol. 2020. PMID: 33195144 Free PMC article. Review.
-
Decellularized Extracellular Matrix for Bioengineering Physiomimetic 3D in Vitro Tumor Models.Trends Biotechnol. 2020 Dec;38(12):1397-1414. doi: 10.1016/j.tibtech.2020.04.006. Epub 2020 May 13. Trends Biotechnol. 2020. PMID: 32416940 Review.
Cited by
-
Understanding fibroblast-immune cell interactions via co-culture models and their role in asthma pathogenesis.Front Immunol. 2023 Feb 23;14:1128023. doi: 10.3389/fimmu.2023.1128023. eCollection 2023. Front Immunol. 2023. PMID: 36911735 Free PMC article. Review.
-
An In Vitro Microfluidic Alveolus Model to Study Lung Biomechanics.Front Bioeng Biotechnol. 2022 Feb 18;10:848699. doi: 10.3389/fbioe.2022.848699. eCollection 2022. Front Bioeng Biotechnol. 2022. PMID: 35252157 Free PMC article.
-
Advanced pathophysiology mimicking lung models for accelerated drug discovery.Biomater Res. 2023 Apr 26;27(1):35. doi: 10.1186/s40824-023-00366-x. Biomater Res. 2023. PMID: 37098610 Free PMC article.
-
Investigation of the role of the autophagic protein LC3B in the regulation of human airway epithelium cell differentiation in COPD using a biomimetic model.Mater Today Bio. 2021 Dec 8;13:100182. doi: 10.1016/j.mtbio.2021.100182. eCollection 2022 Jan. Mater Today Bio. 2021. PMID: 34917923 Free PMC article.
-
Editorial: Innovative In Vitro Models for Pulmonary Physiology and Drug Delivery in Health and Disease.Front Bioeng Biotechnol. 2021 Oct 22;9:788682. doi: 10.3389/fbioe.2021.788682. eCollection 2021. Front Bioeng Biotechnol. 2021. PMID: 34746115 Free PMC article. No abstract available.
References
-
- Prakash YS, Halayko AJ, Gosens R, Panettieri RA, Camoretti-Mercado B, Penn RB, Aiyar R, Ammit A, Berkman N, Bond R, Brown R, et al. An official American thoracic society research statement: Current challenges facing research and therapeutic advances in airway remodeling. Am J Respir Crit Care Med. 2017;195:e4–e19. doi: 10.1164/rccm.201611-2248ST. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

