Efficacy and safety of remdesivir in hospitalised COVID-19 patients: a systematic review and meta-analysis
- PMID: 34331674
- PMCID: PMC8325414
- DOI: 10.1007/s15010-021-01671-0
Efficacy and safety of remdesivir in hospitalised COVID-19 patients: a systematic review and meta-analysis
Abstract
Purpose: This review was aimed to synthesise the best available evidence on the effectiveness and safety of remdesivir in the treatment of moderate to severe COVID-19.
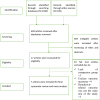
Method: Randomised controlled trials (RCTs) and observational studies reporting the effectiveness and safety of remdesivir were searched via databases and other sources from December 2019 to December 2020. Two independent reviewers performed literature screening, data extraction and assessment of risk bias. Seven studies involving 3686 patients were included.
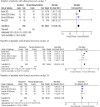
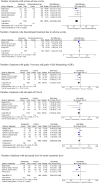
Results: Treatment with remdesivir was associated with an increase in clinical recovery rate by 21% (RR 1.21; 95% CI 1.08-1.35) on day 7 and 29% (RR 1.29; 95% CI 1.22-1.37) on day 14. The likelihoods of requiring high-flow supplemental oxygen and invasive mechanical ventilation in the remdesivir group were lower than in the placebo group by 27% (RR 0.73; 95% CI 0.54-0.99) and 47% (RR 0.53; 95% CI 0.39-0.72), respectively. Remdesivir-treated patients showed a 39% (RR 0.61; 95% CI 0.46-0.79) reduction in the risk of mortality on day 14 compared to the control group; however, there was no significant difference on day 28. Serious adverse effects (SAEs) were significantly less common in patients treated with remdesivir, with an absolute risk difference of 6% (RD -0.06; 95% CI -0.09 to -0.03).
Conclusion: Despite conditional recommendation against its use, remdesivir could still be effective in early clinical improvement; reduction of early mortality and avoiding high-flow supplemental oxygen and invasive mechanical ventilation among hospitalised COVID-19 patients. Remdesivir was also well tolerated without significant SAEs compared to placebo, yet available evidence from clinical studies support the need to conduct close monitoring.
Keywords: Coronavirus deisease-19; Effectiveness; Remdesivir; Safety.
© 2021. Crown.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures



Similar articles
-
Efficacy and safety of remdesivir in hospitalized Covid-19 patients: Systematic review and meta-analysis including network meta-analysis.Rev Med Virol. 2021 Jul;31(4):e2187. doi: 10.1002/rmv.2187. Epub 2020 Oct 31. Rev Med Virol. 2021. PMID: 33128490 Review.
-
Efficacy and harms of remdesivir for the treatment of COVID-19: A systematic review and meta-analysis.PLoS One. 2020 Dec 10;15(12):e0243705. doi: 10.1371/journal.pone.0243705. eCollection 2020. PLoS One. 2020. PMID: 33301514 Free PMC article.
-
Major Update: Remdesivir for Adults With COVID-19 : A Living Systematic Review and Meta-analysis for the American College of Physicians Practice Points.Ann Intern Med. 2021 May;174(5):663-672. doi: 10.7326/M20-8148. Epub 2021 Feb 9. Ann Intern Med. 2021. Update in: Ann Intern Med. 2021 Dec;174(12):W114-W115. doi: 10.7326/L21-0600. Update in: Ann Intern Med. 2022 May;175(5):701-709. doi: 10.7326/M21-4784. PMID: 33560863 Free PMC article. Updated.
-
Remdesivir for treatment of COVID-19; an updated systematic review and meta-analysis.Eur J Pharmacol. 2021 Apr 15;897:173926. doi: 10.1016/j.ejphar.2021.173926. Epub 2021 Feb 4. Eur J Pharmacol. 2021. PMID: 33549577 Free PMC article.
-
Remdesivir for the treatment of COVID-19: a systematic review and meta-analysis.Clin Microbiol Infect. 2022 Sep;28(9):1203-1210. doi: 10.1016/j.cmi.2022.04.018. Epub 2022 May 19. Clin Microbiol Infect. 2022. PMID: 35598856 Free PMC article. Review.
Cited by
-
Population pharmacokinetics and exposure-clinical outcome relationship of remdesivir major metabolite GS-441524 in patients with moderate and severe COVID-19.CPT Pharmacometrics Syst Pharmacol. 2023 Apr;12(4):513-521. doi: 10.1002/psp4.12936. Epub 2023 Feb 22. CPT Pharmacometrics Syst Pharmacol. 2023. PMID: 36798006 Free PMC article.
-
Use of Remdesivir in Patients Hospitalized for COVID-19 Pneumonia: Effect on the Hypoxic and Inflammatory State.Viruses. 2023 Oct 17;15(10):2101. doi: 10.3390/v15102101. Viruses. 2023. PMID: 37896877 Free PMC article.
-
A Randomized Controlled Trial to Evaluate the Safety and Efficacy of a Novel Inhaled Biologic Therapeutic in Adults with Respiratory Distress Secondary to COVID-19 Infection.Infect Dis Ther. 2022 Feb;11(1):595-605. doi: 10.1007/s40121-021-00562-z. Epub 2021 Nov 14. Infect Dis Ther. 2022. PMID: 34775578 Free PMC article.
-
Serum and Urinary Biomarkers in COVID-19 Patients with or without Baseline Chronic Kidney Disease.J Pers Med. 2023 Feb 22;13(3):382. doi: 10.3390/jpm13030382. J Pers Med. 2023. PMID: 36983566 Free PMC article.
-
Remdesivir.Profiles Drug Subst Excip Relat Methodol. 2023;48:71-108. doi: 10.1016/bs.podrm.2022.11.003. Epub 2023 Feb 9. Profiles Drug Subst Excip Relat Methodol. 2023. PMID: 37061276 Free PMC article. Review.
References
-
- World Health Organization. Coronavirus Disease-19 (COVID-19) Dashboard. 2021. https://covid19.who.int/. Accessed 1 Feb 2021.
-
- World Health Organization. Coronavirus disease-2019 (COVID-19) Situation Report. 2021. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situatio.... Accessed 3 Feb 2021.
-
- World Health Organization . Events as they happen. Rolling updates on coronavirus disease-19 (COVID-19) Geneva: World Health Organization; 2020.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

