Human Breast Milk Enhances Intestinal Mucosal Barrier Function and Innate Immunity in a Healthy Pediatric Human Enteroid Model
- PMID: 34327199
- PMCID: PMC8313895
- DOI: 10.3389/fcell.2021.685171
Human Breast Milk Enhances Intestinal Mucosal Barrier Function and Innate Immunity in a Healthy Pediatric Human Enteroid Model
Abstract
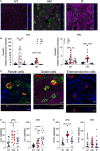
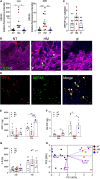
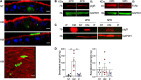
Breastfeeding has been associated with long lasting health benefits. Nutrients and bioactive components of human breast milk promote cell growth, immune development, and shield the infant gut from insults and microbial threats. The molecular and cellular events involved in these processes are ill defined. We have established human pediatric enteroids and interrogated maternal milk's impact on epithelial cell maturation and function in comparison with commercial infant formula. Colostrum applied apically to pediatric enteroid monolayers reduced ion permeability, stimulated epithelial cell differentiation, and enhanced tight junction function by upregulating occludin. Breast milk heightened the production of antimicrobial peptide α-defensin 5 by goblet and Paneth cells, and modulated cytokine production, which abolished apical release of pro-inflammatory GM-CSF. These attributes were not found in commercial infant formula. Epithelial cells exposed to breast milk elevated apical and intracellular pIgR and enabled maternal IgA translocation. Proteomic data revealed a breast milk-induced molecular pattern associated with tissue remodeling and homeostasis. Using a novel ex vivo pediatric enteroid model, we have identified distinct cellular and molecular events involved in human milk-mediated improvement of human intestinal physiology and immunity.
Keywords: breastmilk; enteroid; epithelial barrier; innate immunity; occludin; pIgR polymeric immunoglobulin receptor; pediatric (infant).
Copyright © 2021 Noel, In, Lemme-Dumit, DeVine, Cole, Guerrerio, Campbell, Kovbasnjuk and Pasetti.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Epithelial and Neutrophil Interactions and Coordinated Response to Shigella in a Human Intestinal Enteroid-Neutrophil Coculture Model.mBio. 2022 Jun 28;13(3):e0094422. doi: 10.1128/mbio.00944-22. Epub 2022 Jun 2. mBio. 2022. PMID: 35652591 Free PMC article.
-
Co-Culture System of Human Enteroids/Colonoids with Innate Immune Cells.Curr Protoc Immunol. 2020 Dec;131(1):e113. doi: 10.1002/cpim.113. Curr Protoc Immunol. 2020. PMID: 33166041 Free PMC article.
-
Secretory antibodies in breast milk promote long-term intestinal homeostasis by regulating the gut microbiota and host gene expression.Proc Natl Acad Sci U S A. 2014 Feb 25;111(8):3074-9. doi: 10.1073/pnas.1315792111. Epub 2014 Feb 3. Proc Natl Acad Sci U S A. 2014. PMID: 24569806 Free PMC article.
-
Innate Immunity and Breast Milk.Front Immunol. 2017 May 29;8:584. doi: 10.3389/fimmu.2017.00584. eCollection 2017. Front Immunol. 2017. PMID: 28611768 Free PMC article. Review.
-
Babies, Bugs, and Barriers: Dietary Modulation of Intestinal Barrier Function in Early Life.Annu Rev Nutr. 2022 Aug 22;42:165-200. doi: 10.1146/annurev-nutr-122221-103916. Epub 2022 Jun 13. Annu Rev Nutr. 2022. PMID: 35697048 Review.
Cited by
-
Early human milk feeding: Relationship to intestinal barrier maturation and postnatal growth.Pediatr Res. 2024 Oct 14. doi: 10.1038/s41390-024-03622-5. Online ahead of print. Pediatr Res. 2024. PMID: 39397156
-
Postnatal supplementation with alarmins S100a8/a9 ameliorates malnutrition-induced neonate enteropathy in mice.Nat Commun. 2024 Oct 4;15(1):8623. doi: 10.1038/s41467-024-52829-x. Nat Commun. 2024. PMID: 39366940 Free PMC article.
-
Enteroids to Study Pediatric Intestinal Drug Transport.Mol Pharm. 2024 Oct 7;21(10):4983-4994. doi: 10.1021/acs.molpharmaceut.4c00339. Epub 2024 Sep 16. Mol Pharm. 2024. PMID: 39279643 Free PMC article.
-
Infant and adult human intestinal enteroids are morphologically and functionally distinct.mBio. 2024 Aug 14;15(8):e0131624. doi: 10.1128/mbio.01316-24. Epub 2024 Jul 2. mBio. 2024. PMID: 38953637 Free PMC article.
-
Network analysis of the proteome and peptidome sheds light on human milk as a biological system.Sci Rep. 2024 Mar 30;14(1):7569. doi: 10.1038/s41598-024-58127-2. Sci Rep. 2024. PMID: 38555284 Free PMC article.
References
-
- Aaen K. H., Anthi A. K., Sandlie I., Nilsen J., Mester S., Andersen J. T. (2021). The neonatal Fc receptor in mucosal immune regulation. Scand. J. Immunol. 93:e13017. - PubMed
-
- Andoh A., Fujiyama Y., Bamba T., Hosoda S. (1993). Differential cytokine regulation of complement C3, C4, and factor B synthesis in human intestinal epithelial cell line, Caco-2. J. Immunol. 151 4239–4247. - PubMed
-
- Andoh A., Kinoshita K., Rosenberg I., Podolsky D. K. (2001). Intestinal trefoil factor induces decay-accelerating factor expression and enhances the protective activities against complement activation in intestinal epithelial cells. J. Immunol. 167 3887–3893. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

