Pooled SARS-CoV-2 antigen tests in asymptomatic children and their caregivers: Screening for SARS-CoV-2 in a pediatric emergency department
- PMID: 34314758
- PMCID: PMC8310391
- DOI: 10.1016/j.ajic.2021.07.009
Pooled SARS-CoV-2 antigen tests in asymptomatic children and their caregivers: Screening for SARS-CoV-2 in a pediatric emergency department
Abstract
Background: Universal admission screening for SARS-CoV-2 in children and their caregivers (CG) is critical to prevent hospital outbreaks. We evaluated pooled SARS-CoV-2 antigen tests (AG) to identify infectious individuals while waiting for polymerase chain reaction (PCR) test results.
Methods: This single-center study was performed from November 5, 2020 to March 1, 2021. Nasal mid-turbinate and oropharyngeal swabbing for AG and PCR testing was performed in children with 2 individual swabs that were simultaneously inserted. Nasopharyngeal swabs were obtained from their CG. AG swabs were pooled in a single extraction buffer tube and PCR swabs in a single viral medium. Results from an adult population were used for comparison, as no pooled testing was performed.
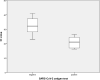
Results: During the study period, 710 asymptomatic children and their CG were admitted. Pooled AG sensitivity and specificity was 75% and 99.4% respectively for detection of infectious individuals. Four false negatives were observed, though 3 out of 4 false negative child-CG pairs were not considered infectious at admission. Unpooled AG testing in an adult population showed a comparable sensitivity and specificity of 50% and 99.7%. AG performed significantly better in samples with lower Ct values in the corresponding PCR (32.3 vs 21, P-value < .001).
Conclusions: Pooled SARS-CoV-2 AGs are an effective method to identify potentially contagious individuals prior admission, without adding additional strain to the child.
Keywords: COVID-19; Hospital infection; Lateral flow test; Mid-turbinate.
Copyright © 2021 Association for Professionals in Infection Control and Epidemiology, Inc. Published by Elsevier Inc. All rights reserved.
Figures
Similar articles
-
Parental Coronavirus Disease 2019 Testing of Hospitalized Children: Rethinking Infection Control in a Pandemic.J Pediatric Infect Dis Soc. 2020 Nov 10;9(5):564-565. doi: 10.1093/jpids/piaa103. J Pediatric Infect Dis Soc. 2020. PMID: 32856705 Free PMC article.
-
Performance Characteristics and Utility of the Standard Q COVID-19 Antigen Test for Emergency Admissions to Healthcare Facilities.Acta Medica (Hradec Kralove). 2022;65(4):139-143. doi: 10.14712/18059694.2023.4. Acta Medica (Hradec Kralove). 2022. PMID: 36942704
-
The sensitivity of SARS-CoV-2 antigen tests in the view of large-scale testing.Epidemiol Mikrobiol Imunol. 2021 Summer;70(3):156-160. Epidemiol Mikrobiol Imunol. 2021. PMID: 34641689 English.
-
Epidemiologic Characteristics Associated With Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Antigen-Based Test Results, Real-Time Reverse Transcription Polymerase Chain Reaction (rRT-PCR) Cycle Threshold Values, Subgenomic RNA, and Viral Culture Results From University Testing.Clin Infect Dis. 2021 Sep 15;73(6):e1348-e1355. doi: 10.1093/cid/ciab303. Clin Infect Dis. 2021. PMID: 33846714 Free PMC article.
-
Current status of the lateral flow immunoassay for the detection of SARS-CoV-2 in nasopharyngeal swabs.Biochem Med (Zagreb). 2021 Jun 15;31(2):020601. doi: 10.11613/BM.2021.020601. Biochem Med (Zagreb). 2021. PMID: 34140830 Free PMC article. Review.
Cited by
-
The Coronavirus Disease 2019 Spatial Care Path: Home, Community, and Emergency Diagnostic Portals.Diagnostics (Basel). 2022 May 12;12(5):1216. doi: 10.3390/diagnostics12051216. Diagnostics (Basel). 2022. PMID: 35626375 Free PMC article.
-
Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) antigen detection in the Emergency Department: data from a pediatric cohort during the fourth COVID-19 wave in Italy.Ital J Pediatr. 2022 Aug 26;48(1):155. doi: 10.1186/s13052-022-01343-1. Ital J Pediatr. 2022. PMID: 36028877 Free PMC article.
References
-
- Robert Koch Institute - Coronavirus SARS-CoV-2 - Testkriterien für die SARS-CoV-2 Diagnostik bei symptomatischen Patienten mit Verdacht auf COVID-19. Available at: https://www.rki.de/DE/Content/InfAZ/N/Neuartiges_Coronavirus/Teststrateg.... Accessed March 23, 2021.
-
- Mina MJ, Parker R, Larremore DB. Rethinking Covid-19 test sensitivity — a strategy for containment. New England J Med. 2020;383:e120. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

