FOXP3 Inhibits the Metastasis of Breast Cancer by Downregulating the Expression of MTA1
- PMID: 34307133
- PMCID: PMC8293273
- DOI: 10.3389/fonc.2021.656190
FOXP3 Inhibits the Metastasis of Breast Cancer by Downregulating the Expression of MTA1
Abstract
Background: FOXP3, as a tumour suppressor gene, has a vital function in inhibiting the metastasis of breast cancer cells, but the mechanisms by which it inhibits metastasis have not been fully elucidated. This study intended to explore a new mechanism by which FOXP3 inhibits breast cancer metastasis.
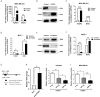
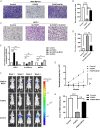
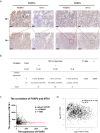
Methods: Bioinformatic analysis was performed to identify potential downstream molecules of FOXP3. The function of FOXP3 in inhibiting MTA1 expression at the mRNA and protein levels was verified by real-time PCR and Western blot analysis. The interaction between FOXP3 and the MTA1 promoter was verified by transcriptomic experiments. In vitro and in vivo experiments were used to determine whether the regulation of MTA1 by FOXP3 affected the invasion and migration of breast cancer cells. Immunohistochemistry was adopted to explore the correlation between the expression levels of FOXP3 and MTA1 in breast cancer samples.
Results: Bioinformatics-based sequencing suggested that MTA1 is a potential downstream molecule of FOXP3. FOXP3 downregulated the expression of MTA1 in breast cancer cells by directly inhibiting MTA1 promoter activity. Importantly, FOXP3's regulation of MTA1 affected the ability of breast cancer cells to invade and metastasize in vitro and in vivo. Moreover, analysis of clinical specimens showed a significant negative correlation between the expression levels of FOXP3 and MTA1 in breast cancer.
Conclusion: We systematically explored a new mechanism by which FOXP3 inhibits breast cancer metastasis via the FOXP3-MTA1 pathway.
Keywords: FOXP3; MTA1; breast cancer; metastasis; transcription factor.
Copyright © 2021 Liu, Han, Li, Huang, Gao, Wang, Zhang, Wang, Zhang, Li, Hao, Li, Zhang and Zhang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
MicroRNA-421 inhibits breast cancer metastasis by targeting metastasis associated 1.Biomed Pharmacother. 2016 Oct;83:1398-1406. doi: 10.1016/j.biopha.2016.08.058. Epub 2016 Aug 29. Biomed Pharmacother. 2016. PMID: 27583980
-
MTA1-TJP1 interaction and its involvement in non-small cell lung cancer metastasis.Transl Oncol. 2022 Nov;25:101500. doi: 10.1016/j.tranon.2022.101500. Epub 2022 Aug 6. Transl Oncol. 2022. PMID: 35944414 Free PMC article.
-
Overexpression of MTA1 inhibits the metastatic ability of ZR-75-30 cells in vitro by promoting MTA2 degradation.Cell Commun Signal. 2019 Jan 14;17(1):4. doi: 10.1186/s12964-019-0318-6. Cell Commun Signal. 2019. PMID: 30642362 Free PMC article.
-
Tumor metastasis-associated human MTA1 gene and its MTA1 protein product: role in epithelial cancer cell invasion, proliferation and nuclear regulation.Clin Exp Metastasis. 2003;20(1):19-24. doi: 10.1023/a:1022534217769. Clin Exp Metastasis. 2003. PMID: 12650603 Review.
-
Emerging roles of MTA family members in human cancers.Semin Oncol. 2003 Oct;30(5 Suppl 16):30-7. doi: 10.1053/j.seminoncol.2003.08.005. Semin Oncol. 2003. PMID: 14613024 Review.
Cited by
-
T-bet Regulates Ion Channels and Transporters and Induces Apoptosis in Intestinal Epithelial Cells.Adv Sci (Weinh). 2024 Jul;11(28):e2401654. doi: 10.1002/advs.202401654. Epub 2024 Apr 22. Adv Sci (Weinh). 2024. PMID: 38650111 Free PMC article.
-
The Potential of FOXP3 in Predicting Survival and Treatment Response in Breast Cancer.Int J Gen Med. 2024 Mar 28;17:1233-1251. doi: 10.2147/IJGM.S454421. eCollection 2024. Int J Gen Med. 2024. PMID: 38562210 Free PMC article.
-
Breast Cancer-Delivered Exosomal miRNA as Liquid Biopsy Biomarkers for Metastasis Prediction: A Focus on Translational Research with Clinical Applicability.Int J Mol Sci. 2022 Aug 19;23(16):9371. doi: 10.3390/ijms23169371. Int J Mol Sci. 2022. PMID: 36012638 Free PMC article. Review.
-
The Role of FOXP3 on Tumor Metastasis and Its Interaction with Traditional Chinese Medicine.Molecules. 2022 Oct 8;27(19):6706. doi: 10.3390/molecules27196706. Molecules. 2022. PMID: 36235242 Free PMC article. Review.
-
A model based on immunogenic cell death-related genes predicts prognosis and response to immunotherapy in kidney renal clear cell carcinoma.Transl Cancer Res. 2024 Jan 31;13(1):249-267. doi: 10.21037/tcr-23-214. Epub 2024 Jan 29. Transl Cancer Res. 2024. PMID: 38410237 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Miscellaneous

