Redox-Active Metal Ions and Amyloid-Degrading Enzymes in Alzheimer's Disease
- PMID: 34299316
- PMCID: PMC8307724
- DOI: 10.3390/ijms22147697
Redox-Active Metal Ions and Amyloid-Degrading Enzymes in Alzheimer's Disease
Abstract
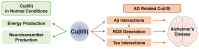
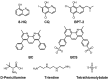
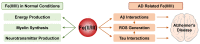
Redox-active metal ions, Cu(I/II) and Fe(II/III), are essential biological molecules for the normal functioning of the brain, including oxidative metabolism, synaptic plasticity, myelination, and generation of neurotransmitters. Dyshomeostasis of these redox-active metal ions in the brain could cause Alzheimer's disease (AD). Thus, regulating the levels of Cu(I/II) and Fe(II/III) is necessary for normal brain function. To control the amounts of metal ions in the brain and understand the involvement of Cu(I/II) and Fe(II/III) in the pathogenesis of AD, many chemical agents have been developed. In addition, since toxic aggregates of amyloid-β (Aβ) have been proposed as one of the major causes of the disease, the mechanism of clearing Aβ is also required to be investigated to reveal the etiology of AD clearly. Multiple metalloenzymes (e.g., neprilysin, insulin-degrading enzyme, and ADAM10) have been reported to have an important role in the degradation of Aβ in the brain. These amyloid degrading enzymes (ADE) could interact with redox-active metal ions and affect the pathogenesis of AD. In this review, we introduce and summarize the roles, distributions, and transportations of Cu(I/II) and Fe(II/III), along with previously invented chelators, and the structures and functions of ADE in the brain, as well as their interrelationships.
Keywords: ADAM10; Cu(I/II); Fe(II/III); amyloid-degrading enzymes; insulin-degrading enzyme; metal chelators; neprilysin; redox-active metal ions.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
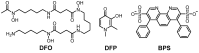
Similar articles
-
Metal Ions in Alzheimer's Disease: A Key Role or Not?Acc Chem Res. 2019 Jul 16;52(7):2026-2035. doi: 10.1021/acs.accounts.9b00248. Epub 2019 Jul 5. Acc Chem Res. 2019. PMID: 31274278
-
Redox-active metals, oxidative stress, and Alzheimer's disease pathology.Ann N Y Acad Sci. 2004 Mar;1012:153-63. doi: 10.1196/annals.1306.012. Ann N Y Acad Sci. 2004. PMID: 15105262 Review.
-
The redox chemistry of the Alzheimer's disease amyloid beta peptide.Biochim Biophys Acta. 2007 Aug;1768(8):1976-90. doi: 10.1016/j.bbamem.2007.02.002. Epub 2007 Feb 9. Biochim Biophys Acta. 2007. PMID: 17433250 Review.
-
Effect of Global Brain Ischemia on Amyloid Precursor Protein Metabolism and Expression of Amyloid-Degrading Enzymes in Rat Cortex: Role in Pathogenesis of Alzheimer's Disease.Biochemistry (Mosc). 2021 Jun;86(6):680-692. doi: 10.1134/S0006297921060067. Biochemistry (Mosc). 2021. PMID: 34225591
-
Copper(I) and copper(II) inhibit Aβ peptides proteolysis by insulin-degrading enzyme differently: implications for metallostasis alteration in Alzheimer's disease.Chemistry. 2011 Feb 25;17(9):2752-62. doi: 10.1002/chem.201002809. Epub 2011 Jan 27. Chemistry. 2011. PMID: 21274957
Cited by
-
Advancements in Portable Voltammetry: A Promising Approach for Iron Speciation Analysis.Molecules. 2023 Nov 3;28(21):7404. doi: 10.3390/molecules28217404. Molecules. 2023. PMID: 37959823 Free PMC article.
-
Inhibition of Insulin Degrading Enzyme to Control Diabetes Mellitus and its Applications on some Other Chronic Disease: a Critical Review.Pharm Res. 2022 Apr;39(4):611-629. doi: 10.1007/s11095-022-03237-7. Epub 2022 Apr 4. Pharm Res. 2022. PMID: 35378698 Review.
-
Metal Nanomaterials and Hydrolytic Enzyme-Based Formulations for Improved Antifungal Activity.Int J Mol Sci. 2023 Jul 12;24(14):11359. doi: 10.3390/ijms241411359. Int J Mol Sci. 2023. PMID: 37511117 Free PMC article. Review.
-
Critical appraisal on mitochondrial dysfunction in Alzheimer's disease.Aging Med (Milton). 2022 Jul 25;5(4):272-280. doi: 10.1002/agm2.12217. eCollection 2022 Dec. Aging Med (Milton). 2022. PMID: 36606272 Free PMC article. Review.
-
Molecular Mechanisms of Obesity-Induced Development of Insulin Resistance and Promotion of Amyloid-β Accumulation: Dietary Therapy Using Weak Organic Acids via Improvement of Lowered Interstitial Fluid pH.Biomolecules. 2023 Apr 30;13(5):779. doi: 10.3390/biom13050779. Biomolecules. 2023. PMID: 37238649 Free PMC article. Review.
References
-
- Savelieff M.G., Nam G., Kang J., Lee H.J., Lee M., Lim M.H. Development of Multifunctional Molecules as Potential Therapeutic Candidates for Alzheimer’s Disease, Parkinson’s Disease, and Amyotrophic Lateral Sclerosis in the Last Decade. Chem. Rev. 2019;119:1221–1322. doi: 10.1021/acs.chemrev.8b00138. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

