Evasion of the Host Immune Response by Betaherpesviruses
- PMID: 34299120
- PMCID: PMC8306455
- DOI: 10.3390/ijms22147503
Evasion of the Host Immune Response by Betaherpesviruses
Abstract
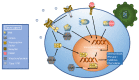
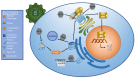
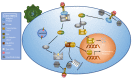
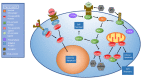
The human immune system boasts a diverse array of strategies for recognizing and eradicating invading pathogens. Human betaherpesviruses, a highly prevalent subfamily of viruses, include human cytomegalovirus (HCMV), human herpesvirus (HHV) 6A, HHV-6B, and HHV-7. These viruses have evolved numerous mechanisms for evading the host response. In this review, we will highlight the complex interplay between betaherpesviruses and the human immune response, focusing on protein function. We will explore methods by which the immune system first responds to betaherpesvirus infection as well as mechanisms by which viruses subvert normal cellular functions to evade the immune system and facilitate viral latency, persistence, and reactivation. Lastly, we will briefly discuss recent advances in vaccine technology targeting betaherpesviruses. This review aims to further elucidate the dynamic interactions between betaherpesviruses and the human immune system.
Keywords: HCMV; HHV-6A; HHV-6B; HHV-7; betaherpesvirus; immune evasion; immune response; viral evasion.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Cell Fusion and Syncytium Formation in Betaherpesvirus Infection.Viruses. 2021 Sep 30;13(10):1973. doi: 10.3390/v13101973. Viruses. 2021. PMID: 34696402 Free PMC article. Review.
-
Entry of betaherpesviruses.Adv Virus Res. 2019;104:283-312. doi: 10.1016/bs.aivir.2019.05.005. Epub 2019 Jun 21. Adv Virus Res. 2019. PMID: 31439151
-
Detection of human Betaherpesvirinae in saliva and urine from immunocompromised and immunocompetent subjects.J Clin Microbiol. 1997 Jun;35(6):1600-3. doi: 10.1128/jcm.35.6.1600-1603.1997. J Clin Microbiol. 1997. PMID: 9163493 Free PMC article.
-
Betaherpesvirus assembly and egress: Recent advances illuminate the path.Adv Virus Res. 2020;108:337-392. doi: 10.1016/bs.aivir.2020.09.003. Epub 2020 Oct 7. Adv Virus Res. 2020. PMID: 33837722 Review.
-
Multiplex qPCR facilitates identification of betaherpesviruses in patients with acute liver failure of unknown etiology.BMC Infect Dis. 2019 Sep 4;19(1):773. doi: 10.1186/s12879-019-4309-4. BMC Infect Dis. 2019. PMID: 31484497 Free PMC article.
Cited by
-
Infections and immune dysregulation in ataxia-telangiectasia children with hyper-IgM and non-hyper-IgM phenotypes: A single-center experience.Front Pediatr. 2022 Oct 20;10:972952. doi: 10.3389/fped.2022.972952. eCollection 2022. Front Pediatr. 2022. PMID: 36340711 Free PMC article.
-
Novel betaherpesviruses and gammaherpesviruses in bats from central China.Sci Rep. 2024 May 9;14(1):10651. doi: 10.1038/s41598-024-61290-1. Sci Rep. 2024. PMID: 38724545 Free PMC article.
-
Mechanisms of T cell evasion by Epstein-Barr virus and implications for tumor survival.Front Immunol. 2023 Dec 21;14:1289313. doi: 10.3389/fimmu.2023.1289313. eCollection 2023. Front Immunol. 2023. PMID: 38179040 Free PMC article. Review.
-
Flavonoids Target Human Herpesviruses That Infect the Nervous System: Mechanisms of Action and Therapeutic Insights.Viruses. 2022 Mar 13;14(3):592. doi: 10.3390/v14030592. Viruses. 2022. PMID: 35336999 Free PMC article. Review.
-
Restructured membrane contacts rewire organelles for human cytomegalovirus infection.Nat Commun. 2022 Aug 11;13(1):4720. doi: 10.1038/s41467-022-32488-6. Nat Commun. 2022. PMID: 35953480 Free PMC article.
References
-
- Ablashi D.V., Berneman Z.N., Kramarsky B., Asano Y., Choudhury S., Pearson G.R. Human herpesvirus-7 (HHV-7) Vivo. 1994;8:549–554. - PubMed
-
- Pellett P.E., Roizman B. Herpesviridae. In: Knipe D.M., Howley P.M., editors. Fields Virology. 6th ed. Lippincott Williams & Wilkins; Philadelphia, PA, USA: 2013. pp. 1802–1822.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

