Nuclear Envelope Integrity in Health and Disease: Consequences on Genome Instability and Inflammation
- PMID: 34298904
- PMCID: PMC8307504
- DOI: 10.3390/ijms22147281
Nuclear Envelope Integrity in Health and Disease: Consequences on Genome Instability and Inflammation
Abstract
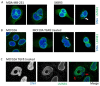
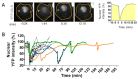
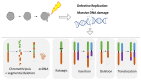
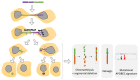
The dynamic nature of the nuclear envelope (NE) is often underestimated. The NE protects, regulates, and organizes the eukaryote genome and adapts to epigenetic changes and to its environment. The NE morphology is characterized by a wide range of diversity and abnormality such as invagination and blebbing, and it is a diagnostic factor for pathologies such as cancer. Recently, the micronuclei, a small nucleus that contains a full chromosome or a fragment thereof, has gained much attention. The NE of micronuclei is prone to collapse, leading to DNA release into the cytoplasm with consequences ranging from the activation of the cGAS/STING pathway, an innate immune response, to the creation of chromosomal instability. The discovery of those mechanisms has revolutionized the understanding of some inflammation-related diseases and the origin of complex chromosomal rearrangements, as observed during the initiation of tumorigenesis. Herein, we will highlight the complexity of the NE biology and discuss the clinical symptoms observed in NE-related diseases. The interplay between innate immunity, genomic instability, and nuclear envelope leakage could be a major focus in future years to explain a wide range of diseases and could lead to new classes of therapeutics.
Keywords: cGAS/STING; cancer; chromosomal instability; envelopathy; inflammation; lipodystrophy; neuropathy; nuclear envelope; nuclear envelope disruption.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Nuclear envelope and chromatin, lock and key of genome integrity.Int Rev Cell Mol Biol. 2015;317:267-330. doi: 10.1016/bs.ircmb.2015.03.001. Epub 2015 Mar 30. Int Rev Cell Mol Biol. 2015. PMID: 26008788 Review.
-
Small but mighty: the causes and consequences of micronucleus rupture.Exp Mol Med. 2020 Nov;52(11):1777-1786. doi: 10.1038/s12276-020-00529-z. Epub 2020 Nov 24. Exp Mol Med. 2020. PMID: 33230251 Free PMC article. Review.
-
cGAS surveillance of micronuclei links genome instability to innate immunity.Nature. 2017 Aug 24;548(7668):461-465. doi: 10.1038/nature23449. Epub 2017 Jul 24. Nature. 2017. PMID: 28738408 Free PMC article.
-
Bursting the Bubble - Nuclear Envelope Rupture as a Path to Genomic Instability?Trends Cell Biol. 2017 Aug;27(8):546-555. doi: 10.1016/j.tcb.2017.02.008. Epub 2017 Mar 9. Trends Cell Biol. 2017. PMID: 28285738 Free PMC article. Review.
-
Understanding the birth of rupture-prone and irreparable micronuclei.Chromosoma. 2020 Dec;129(3-4):181-200. doi: 10.1007/s00412-020-00741-w. Epub 2020 Jul 15. Chromosoma. 2020. PMID: 32671520 Review.
Cited by
-
Type-I Interferon Signaling in Fanconi Anemia.Front Cell Infect Microbiol. 2022 Feb 7;12:820273. doi: 10.3389/fcimb.2022.820273. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 35198459 Free PMC article. Review.
-
Pervasive nuclear envelope ruptures precede ECM signaling and disease onset without activating cGAS-STING in Lamin-cardiomyopathy mice.Cell Rep. 2024 Jun 25;43(6):114284. doi: 10.1016/j.celrep.2024.114284. Epub 2024 May 29. Cell Rep. 2024. PMID: 38814785 Free PMC article.
-
Sculpting nuclear envelope identity from the endoplasmic reticulum during the cell cycle.Nucleus. 2024 Dec;15(1):2299632. doi: 10.1080/19491034.2023.2299632. Epub 2024 Jan 18. Nucleus. 2024. PMID: 38238284 Free PMC article. Review.
-
Nuclear envelope assembly relies on CHMP-7 in the absence of BAF-LEM-mediated hole closure.J Cell Sci. 2023 Nov 1;136(21):jcs261385. doi: 10.1242/jcs.261385. Epub 2023 Nov 13. J Cell Sci. 2023. PMID: 37795681 Free PMC article.
-
Leucocyte Abnormalities in Synovial Fluid of Degenerative and Inflammatory Arthropathies.Int J Mol Sci. 2023 Mar 13;24(6):5450. doi: 10.3390/ijms24065450. Int J Mol Sci. 2023. PMID: 36982526 Free PMC article.
References
-
- Swift J., Ivanovska I.L., Buxboim A., Harada T., Dingal P.C., Pinter J., Pajerowski J.D., Spinler K.R., Shin J.W., Tewari M., et al. Nuclear lamin-A scales with tissue stiffness and enhances matrix-directed differentiation. Science. 2013;341:1240104. doi: 10.1126/science.1240104. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

