Dopamine-dependent early synaptic and motor dysfunctions induced by α-synuclein in the nigrostriatal circuit
- PMID: 34297092
- PMCID: PMC8677552
- DOI: 10.1093/brain/awab242
Dopamine-dependent early synaptic and motor dysfunctions induced by α-synuclein in the nigrostriatal circuit
Abstract
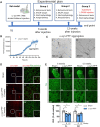
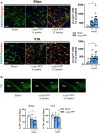
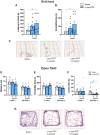
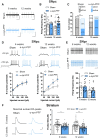
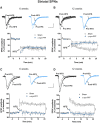
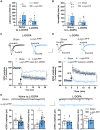
Misfolding and aggregation of α-synuclein are specific features of Parkinson's disease and other neurodegenerative diseases defined as synucleinopathies. Parkinson's disease progression has been correlated with the formation and extracellular release of α-synuclein aggregates, as well as with their spread from neuron to neuron. Therapeutic interventions in the initial stages of Parkinson's disease require a clear understanding of the mechanisms by which α-synuclein disrupts the physiological synaptic and plastic activity of the basal ganglia. For this reason, we identified two early time points to clarify how the intrastriatal injection of α-synuclein-preformed fibrils in rodents via retrograde transmission induces time-dependent electrophysiological and behavioural alterations. We found that intrastriatal α-synuclein-preformed fibrils perturb the firing rate of dopaminergic neurons in the substantia nigra pars compacta, while the discharge of putative GABAergic cells of the substantia nigra pars reticulata is unchanged. The α-synuclein-induced dysregulation of nigrostriatal function also impairs, in a time-dependent manner, the two main forms of striatal synaptic plasticity, long-term potentiation and long-term depression. We also observed an increased glutamatergic transmission measured as an augmented frequency of spontaneous excitatory synaptic currents. These changes in neuronal function in the substantia nigra pars compacta and striatum were observed before overt neuronal death occurred. In an additional set of experiments, we were able to rescue α-synuclein-induced alterations of motor function, striatal synaptic plasticity and increased spontaneous excitatory synaptic currents by subchronic treatment with l-DOPA, a precursor of dopamine widely used in the therapy of Parkinson's disease, clearly demonstrating that a dysfunctional dopamine system plays a critical role in the early phases of the disease.
Keywords: Parkinson’s disease; long-term depression; long-term potentiation; substantia nigra; synaptic plasticity.
© The Author(s) (2021). Published by Oxford University Press on behalf of the Guarantors of Brain.
Figures
Similar articles
-
Depopulation of dense α-synuclein aggregates is associated with rescue of dopamine neuron dysfunction and death in a new Parkinson's disease model.Acta Neuropathol. 2019 Oct;138(4):575-595. doi: 10.1007/s00401-019-02023-x. Epub 2019 May 31. Acta Neuropathol. 2019. PMID: 31165254 Free PMC article.
-
Levodopa enhances synaptic plasticity in the substantia nigra pars reticulata of Parkinson's disease patients.Brain. 2009 Feb;132(Pt 2):309-18. doi: 10.1093/brain/awn322. Epub 2008 Dec 2. Brain. 2009. PMID: 19050033
-
Age-dependent nigral dopaminergic neurodegeneration and α-synuclein accumulation in RGS6-deficient mice.JCI Insight. 2019 May 23;5(13):e126769. doi: 10.1172/jci.insight.126769. JCI Insight. 2019. PMID: 31120439 Free PMC article.
-
Synaptic dysfunction in Parkinson's disease.Adv Exp Med Biol. 2012;970:553-72. doi: 10.1007/978-3-7091-0932-8_24. Adv Exp Med Biol. 2012. PMID: 22351072 Review.
-
A Cortical Pathogenic Theory of Parkinson's Disease.Neuron. 2018 Sep 19;99(6):1116-1128. doi: 10.1016/j.neuron.2018.07.028. Neuron. 2018. PMID: 30236282 Review.
Cited by
-
Simulating combined monoaminergic depletions in a PD animal model through a bio-constrained differential equations system.Front Comput Neurosci. 2024 Aug 23;18:1386841. doi: 10.3389/fncom.2024.1386841. eCollection 2024. Front Comput Neurosci. 2024. PMID: 39247252 Free PMC article.
-
Impaired dopamine release in Parkinson's disease.Brain. 2023 Aug 1;146(8):3117-3132. doi: 10.1093/brain/awad064. Brain. 2023. PMID: 36864664 Free PMC article. Review.
-
Pathophysiological Features of Nigral Dopaminergic Neurons in Animal Models of Parkinson's Disease.Int J Mol Sci. 2022 Apr 19;23(9):4508. doi: 10.3390/ijms23094508. Int J Mol Sci. 2022. PMID: 35562898 Free PMC article. Review.
-
Commentary: Dopamine-Dependent Early Synaptic and Motor Dysfunctions Induced by α-Synuclein in the Nigrostriatal Circuit.Front Aging Neurosci. 2021 Nov 29;13:790224. doi: 10.3389/fnagi.2021.790224. eCollection 2021. Front Aging Neurosci. 2021. PMID: 34912210 Free PMC article. No abstract available.
-
Abscisic acid ameliorates motor disabilities in 6-OHDA-induced mice model of Parkinson's disease.Heliyon. 2023 Jul 20;9(8):e18473. doi: 10.1016/j.heliyon.2023.e18473. eCollection 2023 Aug. Heliyon. 2023. PMID: 37576242 Free PMC article.
References
-
- Spillantini MG, Goedert M.. Synucleinopathies: Past, present and future. Neuropathol Appl Neurobiol. 2016;42(1):3–5. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

