STX2 Promotes Trophoblast Growth, Migration, and Invasion Through Activation of the PI3K-AKT Pathway in Preeclampsia
- PMID: 34295885
- PMCID: PMC8292021
- DOI: 10.3389/fcell.2021.615973
STX2 Promotes Trophoblast Growth, Migration, and Invasion Through Activation of the PI3K-AKT Pathway in Preeclampsia
Abstract
Objectives: Abnormal trophoblast behaviors during pregnancy contribute to the development of preeclampsia (PE). Syntaxin2 (STX2) has been shown to be a crucial epithelial mediator in numerous diseases. However, the functions of STX2 and the mechanisms underlying its role in PE remain largely unknown. The aim of this study was to explore the role of STX2 on trophoblast biology and unravel the molecular mechanisms that contribute to the development and progression of PE.
Materials and methods: We first compared the expression of STX2 in placental tissues from women with PE and women with normal pregnancies. Then, we investigated the role of STX2 on trophoblast proliferation, migration and invasion in HTR-8/SVneo and primary human trophoblast cells by loss or gain of function experiments. In addition, co-immunoprecipitation, pulldown and immunofluorescence assays were performed to investigate the co-localization of STX2 with other proteins, and to help clarify the mechanisms underlying STX2-mediated functions on trophoblasts.
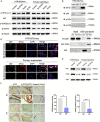
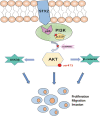
Results: We demonstrated that STX2 expression was downregulated in placental tissues of women with PE compared with those from normal pregnancies. Loss and gain of function experiments further confirmed a role for STX2 in cell proliferation, migration and invasion in trophoblasts. By co-immunoprecipitation, pulldown and immunofluorescence co-localization assays, we revealed that STX2 selectively interacted with p85, a subunit of PI3K, and directly recruited p85 to the cytomembrane, thereby activating the AKT signaling pathway. We further demonstrated that the AKT activation was abolished by the use of a PI3K inhibitor (LY294002), which negatively affected STX2-mediated functions on trophoblasts.
Conclusion: All together, our findings point to a crucial role for STX2 in PE progression. Our new insights also suggest that STX2 may be a potential diagnostic tool and a novel therapeutic target for treating PE.
Keywords: AKT; PI3K; preeclampsia; syntaxin2; trophoblast.
Copyright © 2021 Li, Sun, Ma, Li, Zhan, Li, Li and Wang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Nesfatin-1 Promotes Proliferation, Migration and Invasion of HTR-8/SVneo Trophoblast Cells and Inhibits Oxidative Stress via Activation of PI3K/AKT/mTOR and AKT/GSK3β Pathway.Reprod Sci. 2021 Feb;28(2):550-561. doi: 10.1007/s43032-020-00324-1. Epub 2020 Sep 24. Reprod Sci. 2021. PMID: 32974855
-
Downregulation of receptor tyrosine kinase-like orphan receptor 1 in preeclampsia placenta inhibits human trophoblast cell proliferation, migration, and invasion by PI3K/AKT/mTOR pathway accommodation.Placenta. 2019 Jul;82:17-24. doi: 10.1016/j.placenta.2019.05.002. Epub 2019 May 12. Placenta. 2019. PMID: 31174622
-
ANXA4 promotes trophoblast invasion via the PI3K/Akt/eNOS pathway in preeclampsia.Am J Physiol Cell Physiol. 2019 Apr 1;316(4):C481-C491. doi: 10.1152/ajpcell.00404.2018. Epub 2019 Jan 23. Am J Physiol Cell Physiol. 2019. PMID: 30673304
-
CD97 Is Decreased in Preeclamptic Placentas and Promotes Human Trophoblast Invasion Through PI3K/Akt/mTOR Signaling Pathway.Reprod Sci. 2020 Aug;27(8):1553-1561. doi: 10.1007/s43032-020-00183-w. Reprod Sci. 2020. PMID: 32430705
-
Knockdown of DDX46 inhibits trophoblast cell proliferation and migration through the PI3K/Akt/mTOR signaling pathway in preeclampsia.Open Life Sci. 2020 Jun 13;15(1):400-408. doi: 10.1515/biol-2020-0043. eCollection 2020. Open Life Sci. 2020. PMID: 33817228 Free PMC article.
Cited by
-
Trophectoderm Transcriptome Analysis in LIN28 Knockdown Ovine Conceptuses Suggests Diverse Roles of the LIN28-let-7 Axis in Placental and Fetal Development.Cells. 2022 Apr 5;11(7):1234. doi: 10.3390/cells11071234. Cells. 2022. PMID: 35406798 Free PMC article.
-
Bioinformatic Analysis Identifies Potential Extracellular Matrix Related Genes in the Pathogenesis of Early Onset Preeclampsia.Biochem Genet. 2024 Apr;62(2):646-665. doi: 10.1007/s10528-023-10461-2. Epub 2023 Jul 27. Biochem Genet. 2024. PMID: 37498421
-
Mechanism of histone deacetylase HDAC2 in FOXO3-mediated trophoblast pyroptosis in preeclampsia.Funct Integr Genomics. 2023 May 9;23(2):152. doi: 10.1007/s10142-023-01077-1. Funct Integr Genomics. 2023. PMID: 37160584
-
Upregulation of SEMP1 Contributes to Improving the Biological Functions of Trophoblast via the PI3K/AKT Pathway in Preeclampsia.Mol Biotechnol. 2024 Mar;66(3):531-543. doi: 10.1007/s12033-023-00774-3. Epub 2023 Jun 5. Mol Biotechnol. 2024. PMID: 37277581
-
FPR2 serves a role in recurrent spontaneous abortion by regulating trophoblast function via the PI3K/AKT signaling pathway.Mol Med Rep. 2021 Dec;24(6):838. doi: 10.3892/mmr.2021.12478. Epub 2021 Oct 5. Mol Med Rep. 2021. PMID: 34608500 Free PMC article.
References
-
- Chen C. S., Nelson C. M., Khauv D., Bennett S., Radisky E. S., Hirai Y., et al. (2009). Homology with vesicle fusion mediator syntaxin-1a predicts determinants of epimorphin/syntaxin-2 function in mammary epithelial morphogenesis. J. Biol. Chem. 284 6877–6884. 10.1074/jbc.M805908200 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

